Question
Question: What is the order of increasing heat of hydrogenation for the following compounds (lowest first)? ...
What is the order of increasing heat of hydrogenation for the following compounds (lowest first)?
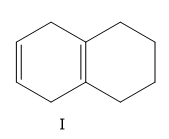
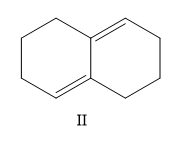
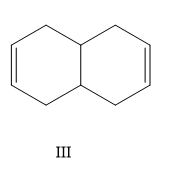
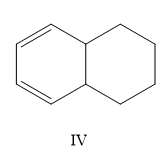
A) I, II, III, IV
B) II, IV, I, III
C) I, III, IV, II
D) IV, II, III, I
Solution
Heat of hydration is the enthalpy or heat released when one mole of an unsaturated system is converted into a saturated system by the addition of excess of hydrogen at normal temperature and pressure.
Complete answer:
So here in the question, we have four unsaturated systems and we have to arrange these systems in the ascending order of their heat of hydrogenation. For solving this question, we should know how to compare the heat of hydrogenation and the stability of the double bonds and how they are related.
First let’s see what does heat of hydrogenation refer to. Heat of hydrogenation is a parameter of how much energy or heat is released during the reaction between a mole of unsaturated system and excess of hydrogen.
When excess of hydrogen is added to an unsaturated system with proper conditions, the system will be converted into a saturated system which is more stable.
We simply can't say that heat of hydrogenation gives a measure of the stability of the double bonds or the alkenes. The heat of hydrogenation is inversely proportional to the stability of the alkenes.
Heatofhydrogenation=stabilityofalkenes1
Let’s move on to the question, and let’s compare the structures as two sets.
Here the structure (II) and structure (III) is a conjugated diene structure and it possesses a linear resonance. We know that the conjugated dienes are having higher stability than the normal dienes. So these two structures are more stable than other options.
Now, which one is more stable is the question. The most substituted structure will have the least heat of hydrogenation or high stability. So II < IV.
Now consider the other two options, here I is more substituted than III so I will have less value of heat of hydrogenation than III.
So the correct order is II < IV < I < III
Hence the correct option is option (B).
Note:
We should consider all the factors that affect the stability of the structure, if any substituents are present then effects like Inductive effects, resonance effects etc. should be considered.
By knowing the value of the heat of hydrogenation we could comment on the stability of the molecule.
Heat of hydrogenation is an exothermic process.
