Question
Question: What is the orbital hybridization theory?...
What is the orbital hybridization theory?
Solution
Orbital hybridization means there is the mixing of the orbital of the atoms to form the molecule. For example, there are four sp3 hybridized orbitals in the methane molecule as there are four bonds in the methane molecule.
Complete answer:
There are many theories that were used to define the bonding formed in the molecule, so the orbital hybridization theory is one of them. According to this theory, the atomic orbitals of the atoms combine to form the molecular orbitals. These molecular orbitals are formed so that the shape and energy of the orbitals become equal as the atomic orbitals of the different atoms have different shapes and energy. The formed molecular orbitals having the same shape and energy can overlap each other to form the chemical bond.
For example, the formation of methane (CH4) can be explained in this theory. We know that all four bonds are in the shape of tetrahedral in the methane molecule.
The electronic configuration of the carbon atom before the bond formation will be:
1s22s22p2
This is shown as:
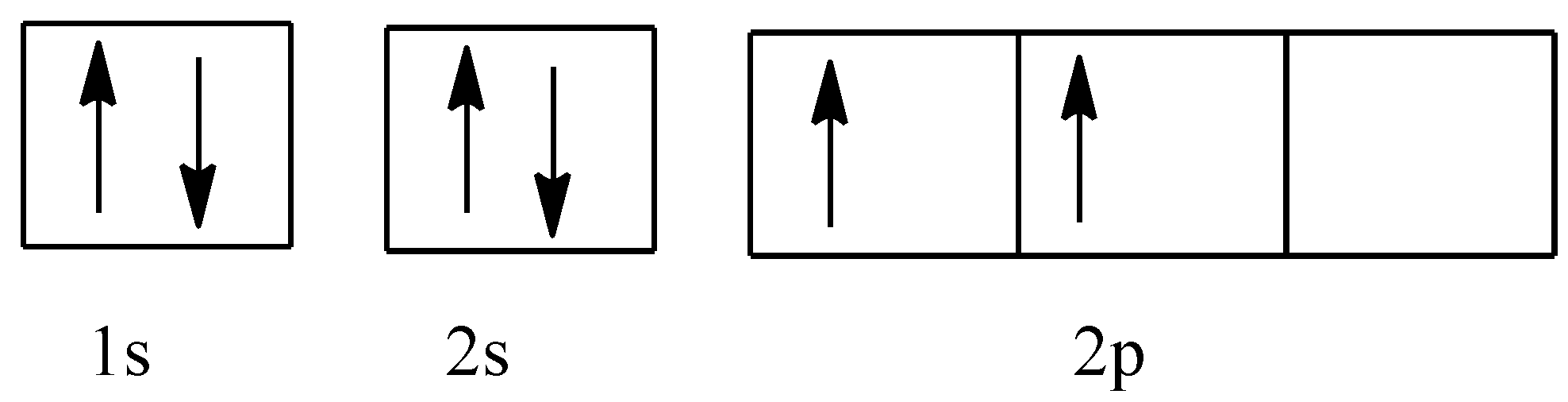
And the configuration of the hydrogen atom will be:
1s1
The electronic configuration of carbon atom when the mixing of the orbital takes place will be:
1s22s12px12py12pz1
This is shown as:
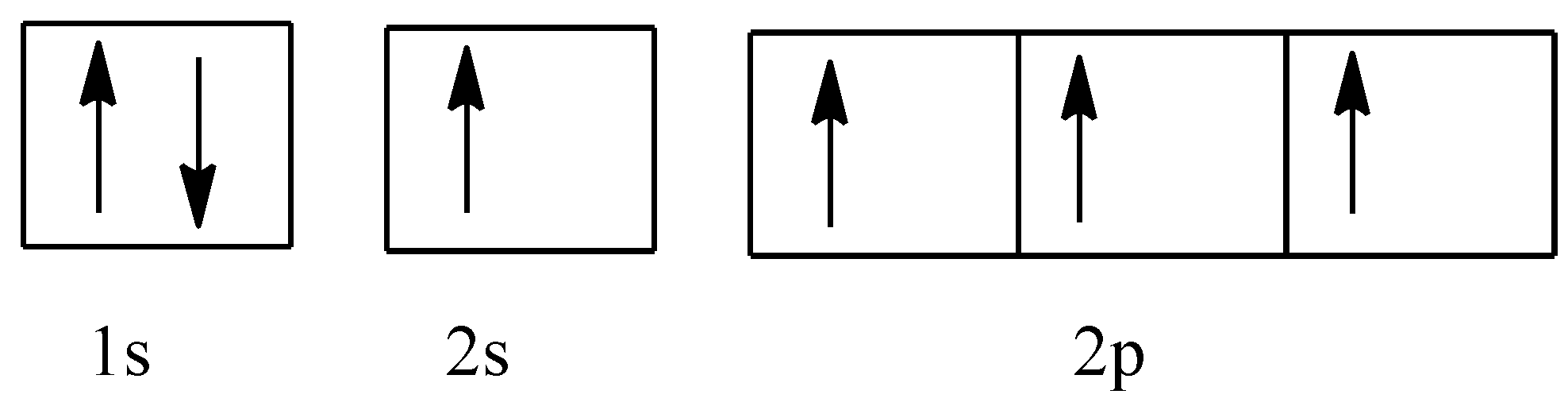
So, after the mixing, all the four orbitals will have the same energy, and they will be termed as sp3 orbital.
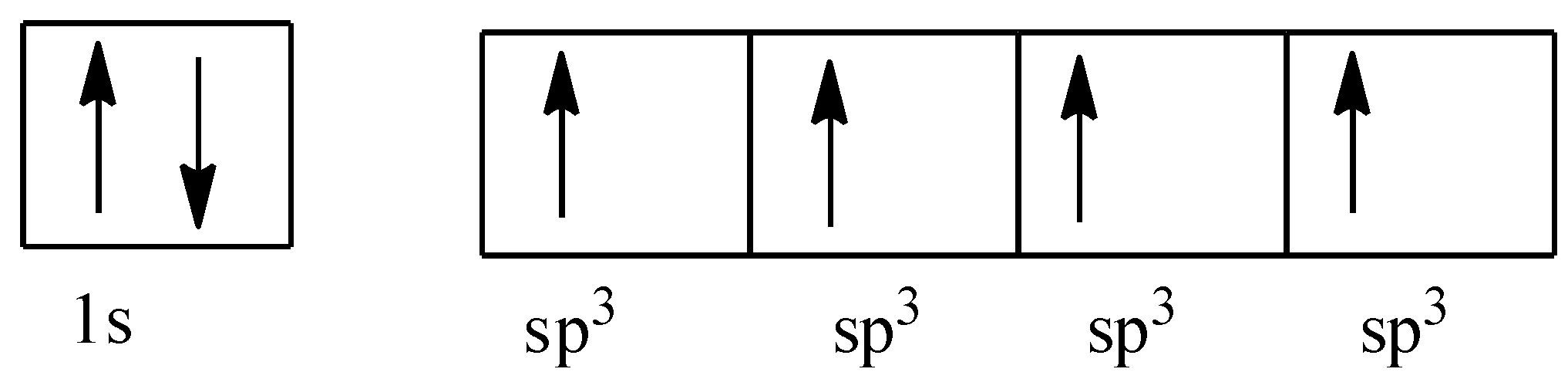
All the orbitals of the hydrogen atom will combine with the sp3 orbitals of the carbon atoms. So, the length of the bonds is the same and the energy is also the same. The structure is given below:
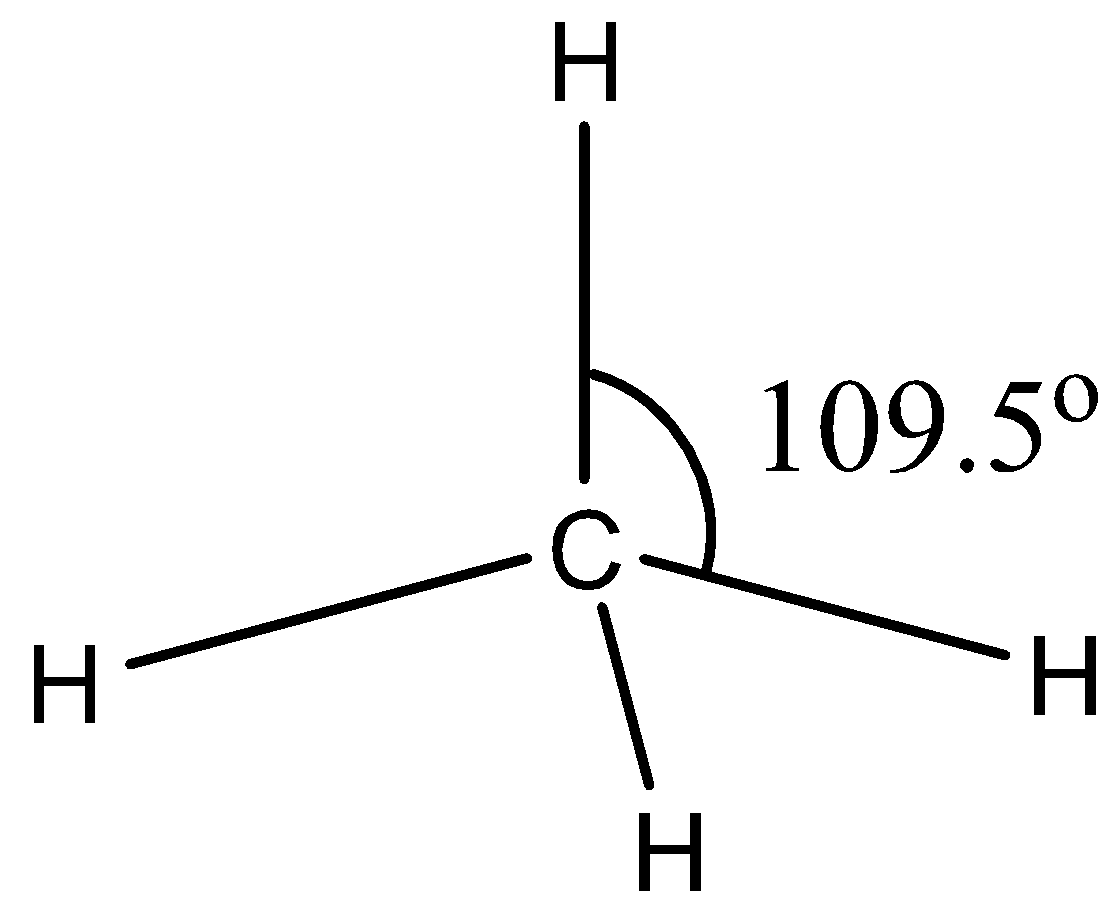
Note:
With the help of the hybridization calculated by the orbital hybridization theory can directly use to find the structure like, if the hybridization is sp then the shape will be linear, if the hybridization is sp2 then the structure is trigonal planar, etc.
