Question
Question: What is the number of \[\sigma \] bonds in product P? \(C{H_3} - Cl + KCN \to P\)...
What is the number of σ bonds in product P?
CH3−Cl+KCN→P
Solution
We need to remember that we first predict the product P and then proceed to understand the concept of σ bonds and how to calculate them. The reaction given is a reaction between methyl chloride and potassium cyanide. It is a reaction between a haloalkane and a nucleophile. In addition, to study about σ bonds we need to understand molecular orbitals and molecular orbital theory.
Complete step by step answer:
We need to know that when methyl chloride reacts with potassium cyanide to undergo a nucleophilic substitution reaction to form methyl cyanide and potassium chloride. We can write the chemical reaction for this reaction is as follows:
CH3−Cl+KCN→CH3−CN+KCl
To begin understanding molecular orbitals under the molecular orbital theory, we have to first consider atomic orbitals. Let us consider the example of the simplest atom-hydrogen. Two isolated hydrogen atoms have one electron located in the 1s subs hell when it is in ground state (as per Aufbau principle) and these electrons of course do not move in perfect circles around the nucleus but they do define a region of a space defined by a sphere. But when two atomic orbitals combine to form a chemical bond. In case of hydrogen molecule (H2) ,the two 1s sub shells combine and form a region of space known as a sigma (σ) molecular orbital where the electrons generally live in between the two hydrogen nuclei, screening the repulsive positive charges they have on one another and stabilize keeping the two nuclei close together. Hence we can say that when more than one atom is involved, we refer to these orbitals as molecular orbitals.
We have to remember that the first covalent bond between two atoms is always a sigma bond (σ) . Any second or third bond is known as a pi bond. Let us explain this with the help of a simple HCN molecule. This molecule has one single and one triple bond given as follows H−C≡N . As we know that the first covalent bond is always a sigma bond, the single bond between H and C is definitely a sigma bond and the first bond between C and N is a sigma bond and the remaining two are pi bonds.
Now let us calculate the number of sigma bonds of the products of the reactionCH3−Cl+KCN→CH3−CN+KCl.
For CH3−CN, the skeletal structure is to be drawn to evaluate the bonds between atoms:
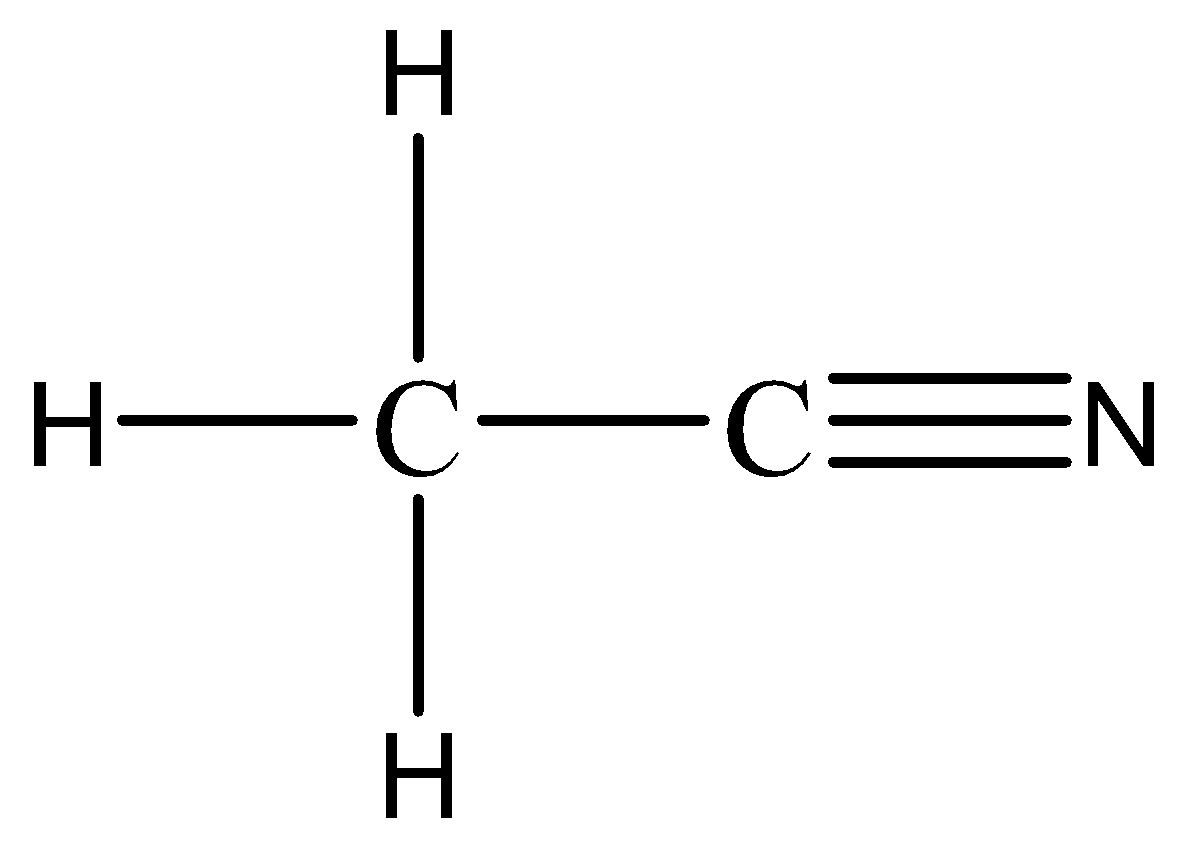
As seen in the skeletal structure, there are 4 single bonds and one triple bond. Hence the 4 single bonds are sigma bonds along with the first bond of the triple bond.
Hence, the total number of σ bonds in CH3−CN is equal to 5.
For KCl, the skeletal structure is a single bond between the two atoms as K−Cl .Hence the number of sigma bonds in this product is equal to 1.
Note: We must be noted that there is a simple trick to calculate the number of sigma bonds in a molecule. The formula for this trick is S=x−1 where S is the number of sigma bonds and x is the number of atoms in the molecule. The number of molecules is CH3−CN is 6. According to the formula, x=6 . Therefore, S=6−1=5 sigma bonds.
