Question
Question: What is the Number of possible stereoisomers in C 
(A) 2
(B) 3.
(C) 4
(D) 5
Solution
Hint : We know that isomers are the molecules which have the same molecular formula but different structural formula. Mainly isomers are divided into two types- the structural isomers and the geometrical or stereoisomers. So, stereoisomers are the ones which have the same composition (or same parts) but those parts differ when oriented in space. Further stereoisomers are divided into diastereomers and enantiomers.
Complete Step By Step Answer:
In the first step we will convert butan−2−ol into butan−2−one as PCC is an oxidizing agent which converts alcohols into aldehydes or ketones but is not strong enough to further convert them into carboxylic acid so the reaction will stop when an aldehyde or a ketone is formed.
So, the conversion equation will be: -
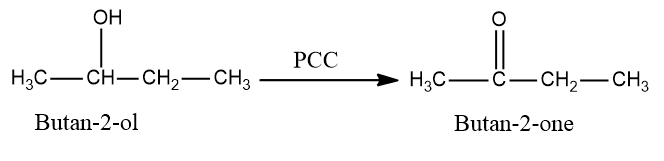
Now in the next step selenium dioxide is used to convert butan−2−one into butanedione as SeO2 is also an oxidizing agent which converts alkenes into alcohols and further converts alcohol into aldehydes or ketones.
So, the conversion equation will be: -
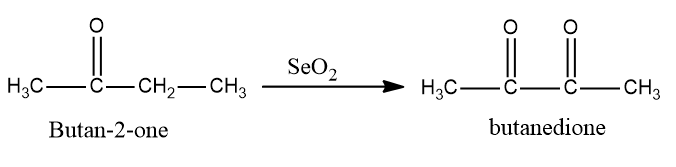
Now we see that butanedione will be converted into but butan−2−ene is a reducing agent and is being added in the last step. LiAlH4 is a reducing agent and will first convert ketone into alcohol and then into alkene.
So, the conversion equation will be: -
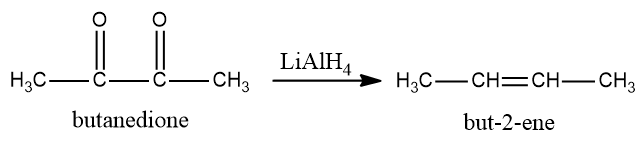
Therefore, in the given equation: -
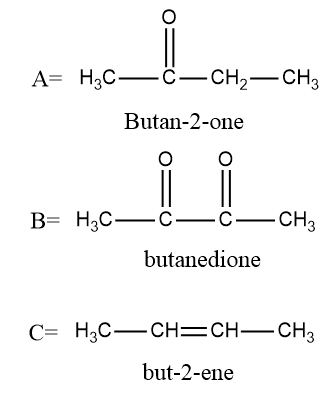
To determine the number of stereoisomers possible in butan−2−ene we will draw the structures
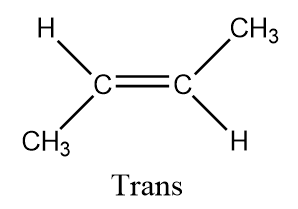
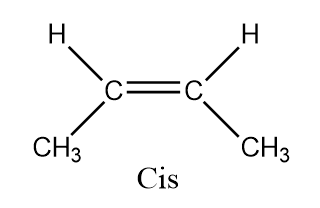
Hence there are two types of ismores possible in this and hence it forms Cis - butan−2−ene and Trans - butan−2−ene
So, the correct answer is Option A.
Note :
Here butan−2−ene is a diastereomer. Diastereomers are stereoisomers which are not the mirror images of one another and are not superimposable on one another. Also, cis isomers are the isomers those have the two single hydrogen atoms present on the same side while trans have the two single hydrogen atoms present on the opposite sides.
