Question
Question: What is the number of aromatic isomers for \(C_{7}H_{8}O\) ?...
What is the number of aromatic isomers for
C7H8O ?
Solution
Isomerism is a phenomenon of organic chemistry in which more than one species has the same chemical formula but different structures. Those compounds that have the same chemical formula but different properties and the arrangement of atoms in their molecule are called isomers.
Complete answer:
- In aromatic isomerism, the basic structure is benzene whose molecular formula is C6H6 and the rest of the atoms are placed differently on the ring forming different isomers with the same atoms.
- Here we are given the molecular formula C7H8O and the benzene ring formula which is C6H6, so the remaining atoms we have is one carbon, two hydrogen and one oxygen atom.
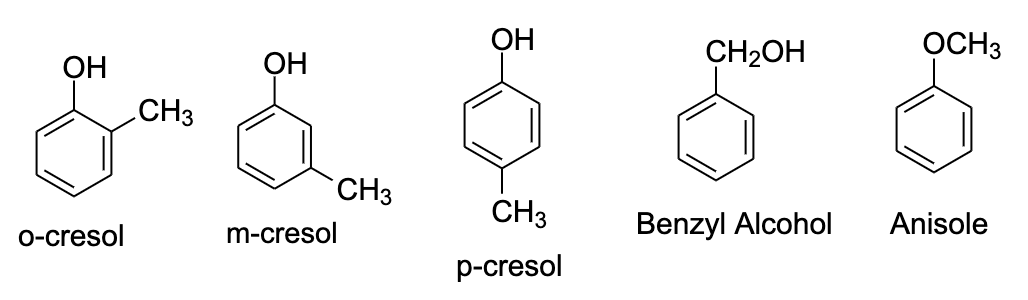
- C7H8 is toluene. It’s the only benzenoid isomer that is possible. Now we have to insert one oxygen atom into the ring. This can be done in five ways.
- We can arrange the CH3 and O atom on the ortho, para, and meta position, that will get us 2-methylphenol, 3-methylphenol, and 4-methylphenol. The common names of these compounds are o-cresol, m-cresol, and p-cresol.
- One way is that if we place the oxygen atom with the methyl group which will lead to the formation of methyl phenyl ether also known as anisole.
- The fifth isomer could be benzyl alcohol in which the −OH group is attached to the CH2 group.
- Hence, in this way, we can say that the possible isomers for the given molecular formula will be five. These isomers are shown below:
Note:
Isomerism is of two types; structural and stereo. Aromatic isomers is a type of structural isomerism. In structural isomerism, the molecular formula of every compound is the same but has different placement of bonds and atoms in the molecule.
