Question
Question: What is the normality of 0.2M \[{H_3}P{O_3}\] solution? A.0.2 N B.0.1 N C.0.4 N D.0.6 N...
What is the normality of 0.2M H3PO3 solution?
A.0.2 N
B.0.1 N
C.0.4 N
D.0.6 N
Solution
We need to know the concept of Basicity of an acid to solve this question. It is the number of hydrogen ions which can be produced from one molecule of the acid. For example Acetic acid is acid which means that as it can lose one proton or hydrogen atom, it forms an acetate ion. H3PO3 is a dibasic acid which is evident from its structure.
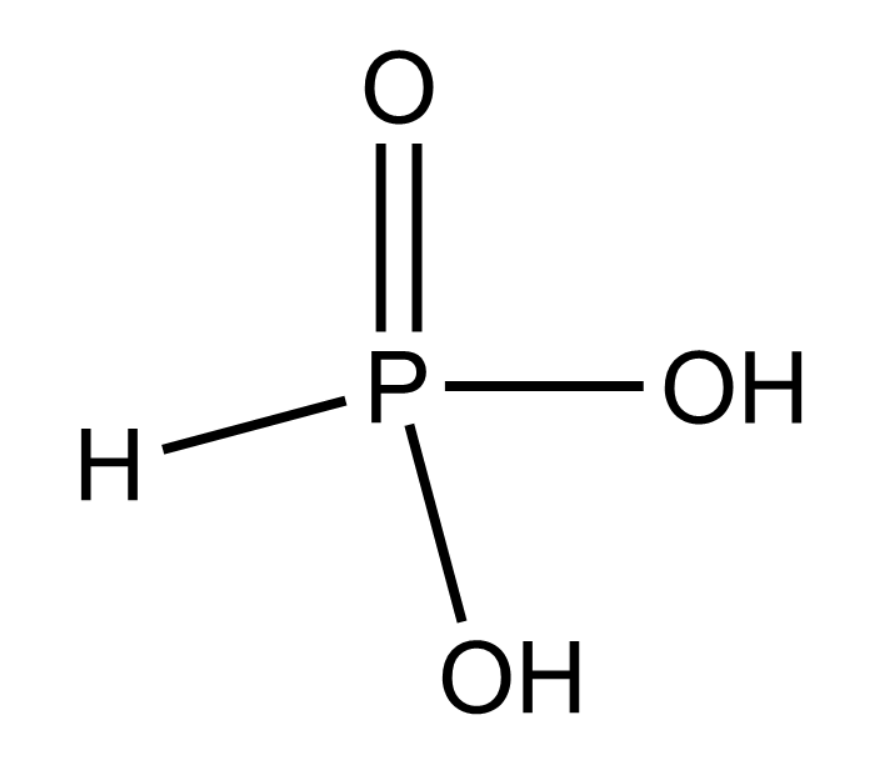
Complete answer:
From the figure given above we can say that H3PO3 is a dibasic acid because both of the −OH group can easily lose its hydrogen as H+ ions making it acidic
We also know that normality can be expressed by the formula
⇒Normality=Molarity×n factor
Where n factor is the number of replaceable H+ ions in that molecule.
Since we are given H3PO3 we can say that the value of n factor will be 2
It is also given to us that the molarity of the given solution is 0.2 M.
Thus we can write that
⇒Normality=0.2M×2
⇒Normality=0.4 N
Thus we can say that the normality of the given solution of 0.2M H3PO3 is 0.4 N
Thus the correct option is C .
Note:
For acids we can say that n factor is the number of replaceable H+ ions in that molecule.
For bases we can say that n factor is the number OH− of ions that the molecule of base would give when dissolved in a suitable solvent.
For Redox reactions n factor will be the change in the oxidation number of the reducing or oxidising agent
