Question
Question: What is the name of the product of the hydrogenation of \[2 - methylpropanal\] ?...
What is the name of the product of the hydrogenation of 2−methylpropanal ?
Solution
We have to remember that in chemistry two reactions are mainly in organic concepts. The two reactions are oxidation and reduction reaction. The oxidation reaction is nothing but a chemical reaction from the reactant to produce the addition of oxygen or removal of hydrogen or gain of electron in the product. The reduction reaction is nothing but a chemical reaction from the reactant to produce the addition of hydrogen or removal of oxygen or loss of electrons. The oxidation or reduction reaction plays a key role for converting one functional group to another functional group in the organic chemical reaction.
Complete step by step answer:
We must have to know that the hydrogenation is nothing but the reduction reaction in the chemistry.
The chemical for of 2−methylpropanal is
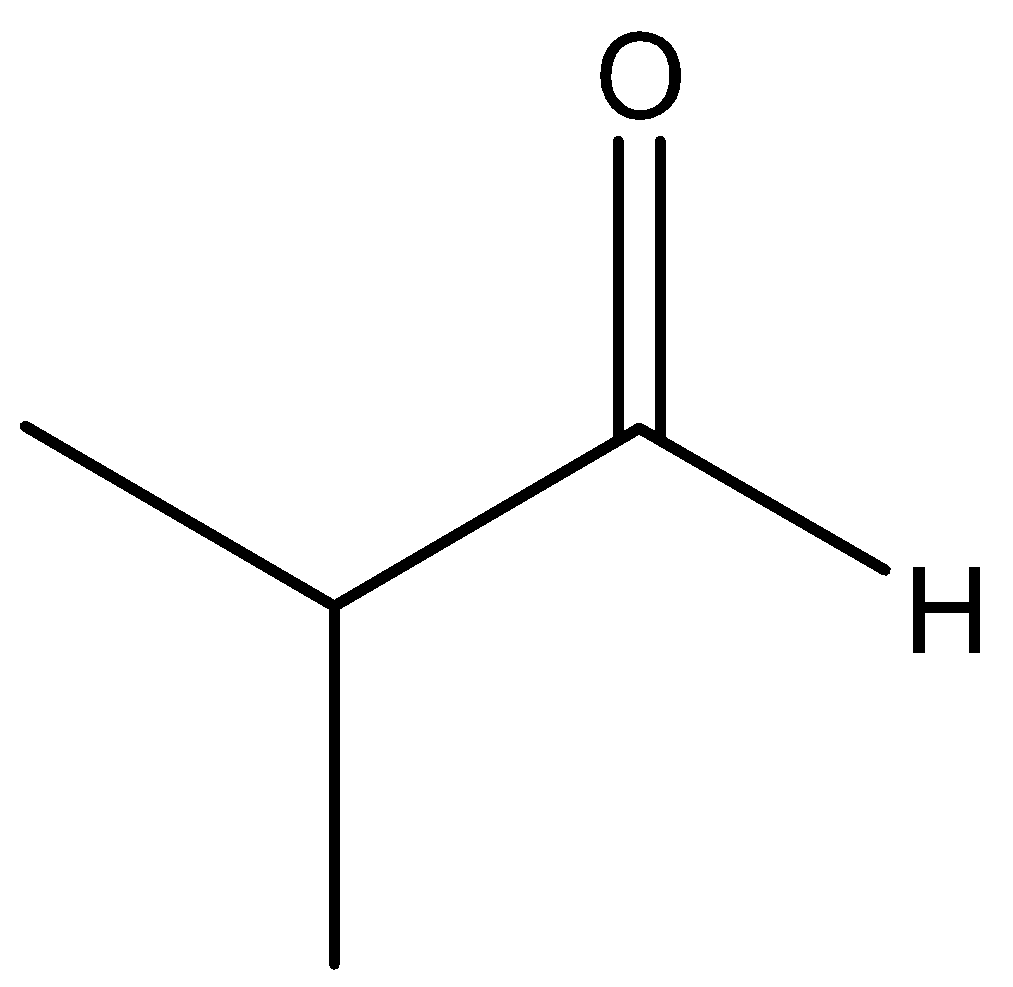
The function group present in the 2−methylpropanal is aldehyde. Reduction of the aldehyde group gives respect to alcohol. Maximum reduction of aldehyde gives primary alcohol in organic chemistry.
The hydrogenation of 2−methylpropanal , hydrogen attacks the carbonyl group in the molecule. Here, the carbonyl group is in the form of aldehyde, reduction of these aldehydes give 2−methylpropanol .
The reaction scheme of the above discussion is given below,
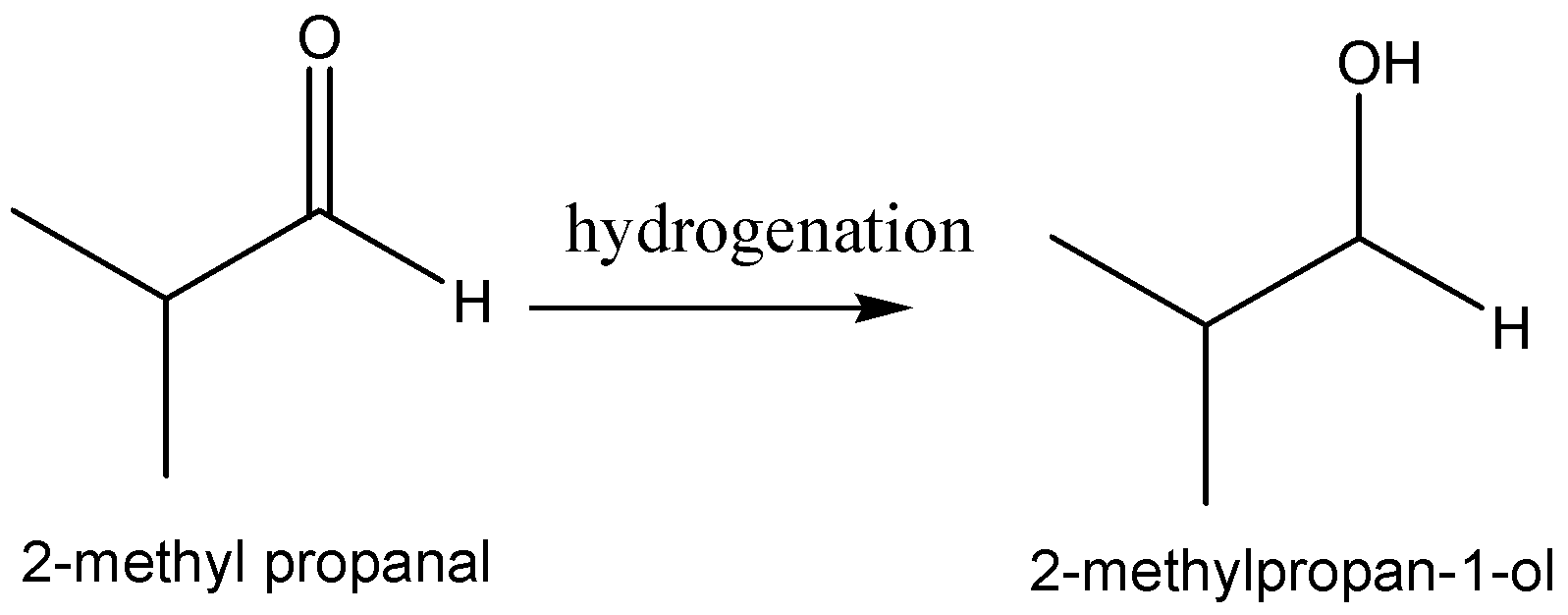
Hence, the product of the hydrogenation of 2−methylpropanal is 2−methylpropanal.
Note: The hydrogenation means two hydrogen atoms are added in the reactant to give product after attacking these two hydrogens. The maximum two hydrogen are attacking in the electrophilic group in the reactant by using some specific reducing reagent. Here, lithium aluminium hydride or any metallic hydride used to convert aldehyde to alcohol group in 2−methylpropanal . The oxidation and reduction are the reversible reactions in organic chemistry. For example, the oxidation of primary alcohol is aldehyde. At the same time, the reduction of aldehyde is primary alcohol. Hence, the oxidation of primary alcohol and reduction of aldehyde is a reversible reaction.
