Question
Question: What is the name of the following reaction? \[\begin{aligned} & \text{C}{{\text{H}}_{\text{3}}...
What is the name of the following reaction?
& \text{C}{{\text{H}}_{\text{3}}}\text{-Br}\,\text{+}\,\,\text{AgF}\,\,\to \,\,\text{C}{{\text{H}}_{\text{3}}}\text{F}\,\,\text{+}\,\,\text{AgBr} \\\ & \\\ \end{aligned}$$ (A) Sandmeyer Reaction (B) Gatterman Reaction (C) Finkelstien Reaction (D) Swarts ReactionSolution
Sandmeyer, Gatterman, Finkelstien and Swart reaction follow the nucleophilic substitution reaction. Sandmeyer and Gatterman reaction are the preparatory methods of aromatic halide, while Finkelstien and Swart reaction are the preparatory methods of aliphatic halide.
Complete Solution :
(A) Sandmeyer reaction - this is a preparatory method of aryl halide, by the decomposition of benzenediazonium chloride. In this nucleophilic substitution reaction substitution of the diazo group takes place by halogen atom. This reaction is catalysed by halide salt of copper, as a result formation of aromatic halide and nitrogen gas take place.
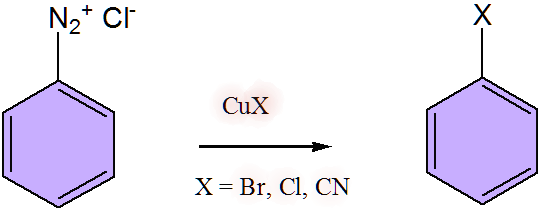
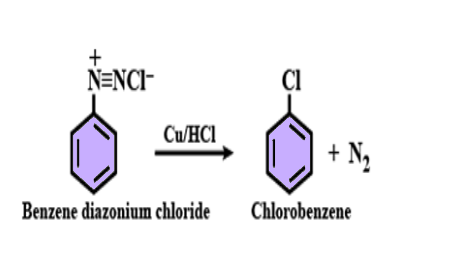
(B) Gatterman reaction - this is also a preparatory method of aromatic halide. In this reaction diazonium salt is reacted withHCl and HBr in the presence of copper metal, as a result haloarene (chlorobenzene or bromobenzene) and N2 gas is formed.
(C) Finkelstien reaction -this is a bimolecular nucleophilic substitution reaction for the preparation of one alkyl halide to other alkyl halide in the presence of dry acetone.
R-XNaIdryacetoneR-I+NaX(X=Cl,Br)
This reaction is mainly preferred for the synthesis of alkyl iodide. In this reaction lower alkyl halide (X=Cl,Br) reacts with sodium iodide in the presence of dry acetone. As a result alkyl iodide forms.
(D) Swart reaction - this reaction is mainly used for the synthesis of alkyl fluoride. This is a bimolecular substitution reaction in which alkyl chloride or bromide is heated in the presence of metallic fluoride such as AgF,Hg2F2andCaF2.
CH3-Br+AgF→CH3F+AgBr
Note: 1∘ halide undergo SN2 reaction except when a strong hindered base is used, and 3∘ alkyl halide undergo SN1 reaction in the absence of a strong base.
