Question
Question: What is the name of the compound \( {(N{H_4})_3}P{O_4} \) ?...
What is the name of the compound (NH4)3PO4 ?
Solution
Hint : (NH4)3PO4 is an unstable inorganic compound made up of ammonium and phosphate salt. Its molecular mass is 149.09g/gmolmol and density 1.619g/gcubiccmcubiccm , chemical formula H9N2O4P .
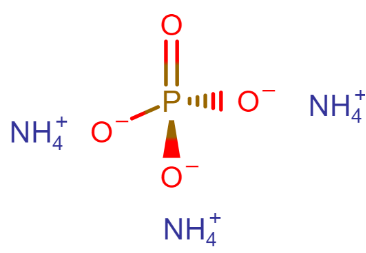
Complete Step By Step Answer:
The name of the compound Is Ammonium phosphate, other names- triammonium phosphate, diazonium hydrogen phosphate, are stable materials that are commonly used as fertilizers to provide plants with fixed nitrogen and phosphorus.
Triammonium phosphate can be prepared in the laboratory by treating 85% phosphoric acid with 30% ammonia solution:
H3PO4+3NH3→(NH4)3PO4
(NH4)3PO4 is a colorless, crystalline solid. The solid, which has the odor of ammonia, is readily soluble in water. The salt converts to diammonium hydrogen phosphate (NH4)3HPO4 .
Chemical properties:
∙ Ammonium phosphate readily undergoes decomposition reaction emitting very toxic fumes. It forms phosphoric acid and ammonia.
(NH4)3PO4→3NH3+H3PO4
∙ Ammonium phosphate reacts with lead nitrate forming lead phosphate and ammonium nitrate.
4(NH4)3PO4+3Pb(NO3)4→Pb3(PO4)4+12NH4NO3
Ammonium phosphate is soluble in water, and ammonia loses and the acid phosphate (NH4) (H2PO4) is formed as the aqueous solution on the boil. Ammonium phosphate is a high source of elemental nitrogen used as an ingredient in certain fertilizers, this is also used in thermoplastic formulations as a flame retardant.
Uses:
Ammonium phosphate is a broad generic name for a variety of fertilizers containing both nitrogen and phosphate. Used as components of intumescent paints and mastics where they function as an acid catalyst. Used in paints.
Note :
Ammonium phosphate is also manufactured by mixing together ammonium phosphate and urea in a molten condition. Considerable heat is generated which transforms the ammonium phosphate sulphate to the molten state. It includes a group of nitrogen phosphorus materials, mono ammonium phosphates, mixtures of the two or combination with ammonium nitrate or ammonium sulfate.
