Question
Question: What is the name of \({H_3}P{O_3}\) ?...
What is the name of H3PO3 ?
Solution
Phosphorus forms oxyacids with oxygen. All these compounds contain phosphorus atoms linked tetrahedrally to four other atoms or groups. Each of them has at least one P→O unit and one P−OH group.
Complete step by step answer:
-Phosphorus forms a number of oxyacids. Each of them has at least one P→O unit and one P−OH group. The −OH group is ionisable but hydrogen directly linked to phosphorus is not ionisable. Thus the number of −OH group decides the basicity of oxyacids.
-The given compound is also an oxyacid of phosphorus. It is called phosphorus acid. The phosphorus atom in this compound is sp3 hybridized. The structure of phosphorus acid can be represented as follows-
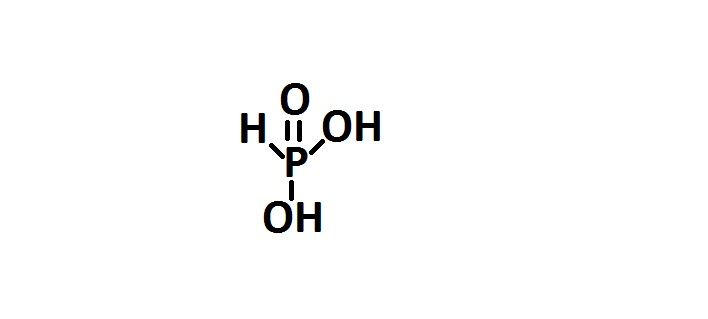
-The two hydrogens are ionisable. The oxidation state of phosphorus atoms is +3. It is also called phosphonic acid.
-It is prepared by dissolving phosphorus trioxide in water.
P4O6+6H2O→4H3PO3
-It can also be obtained by hydrolysis of phosphorus trichloride.
PCl3+3H2O→H3PO3+3HCl
It is a crystalline colorless compound. It is highly soluble in water.
-When phosphorus acid is heated, it disproportionates into phosphoric acid and phosphine.
4H3PO3200∘C3H3PO4+PH3
It acts as a strong reducing agent. It reduces CuSO4 to Cu, AgNO3 to Ag, I2 to HI.
CuSO4+2H→Cu+H2SO4
AgNO3+H→HNO3+Ag
I2+2H→2HI
So the name of H3PO3 is phosphorus acid or phosphonic acid.
Additional information:
Some other oxyacids of phosphorus are-
H3PO2 - Hypophosphorous acid
H3PO4 - Orthophosphoric acid
HPO3 - Metaphosphoric acid
H4P2O6 - Hypophosphoric acid
H4P2O7 - Pyrophosphoric acid
Note: The hydrogen directly attached to phosphorus in H3PO3 is not ionisable. It is a diprotic oxyacid of phosphorus. It is used as a starting material for the synthesis of many phosphorus compounds. It is used as a strong reducing agent.
