Question
Question: What is the name of \({H_3}P{O_3}\) ?...
What is the name of H3PO3 ?
Solution
In H3PO3 , the hydrogen is bonded directly to the central phosphorus atom. It is prepared by dissolving tetraphosphorus hexoxide (P4O6) or Phosphorous trichloride (PCI3) in water.
Complete step by step answer:
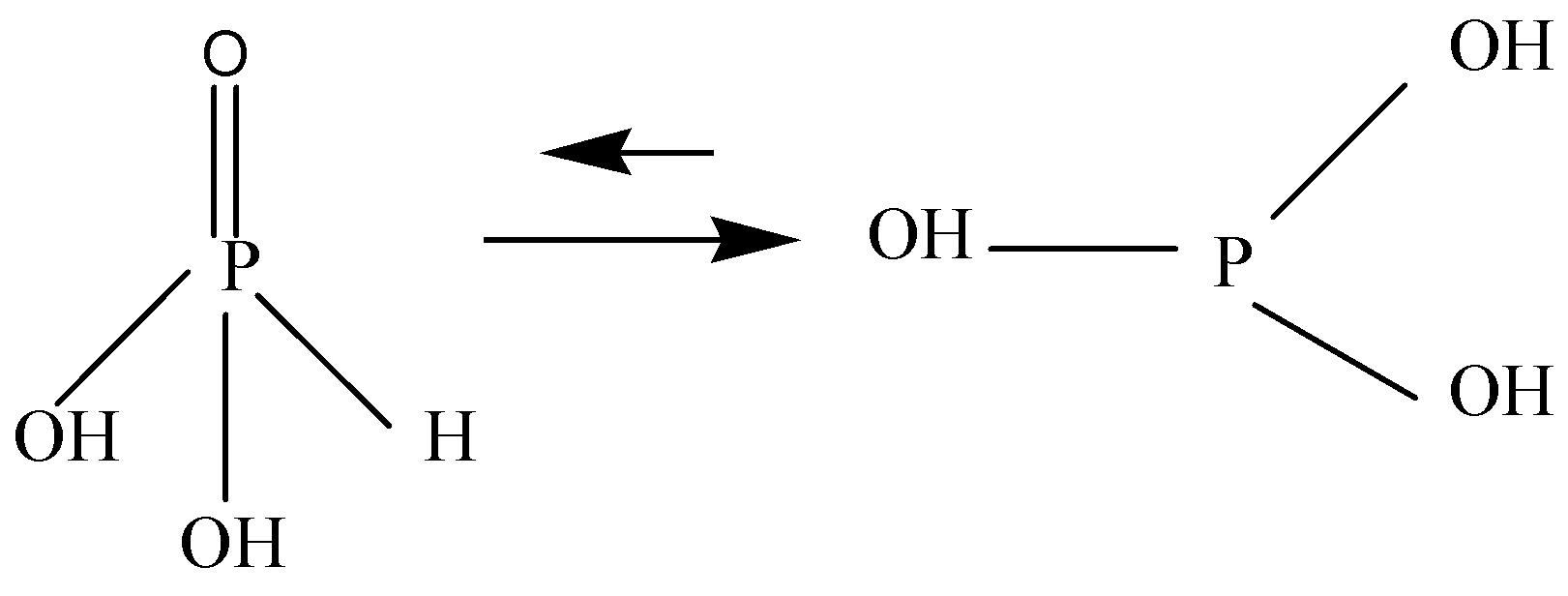
H3PO3 is ambiguous. It is diprotic and called Phosphorus acid or Orthophosphoric acid, having two hydroxyl groups, one doubly bonded oxygen and one hydrogen atom attached to centrally placed pentavalent phosphorus. It is soluble in water.
Phosphorus acid forms salts called Phosphites, also used as a reducing agent. It is the conjugate acid of Phosphite anion.
It is prepared by dissolving tetraphosphorus hexoxide (P4O6) or Phosphorous trichloride (PCI3) in water. Phosphorous acid is one of several oxygen acids of Phosphorus.
H3PO3 is not stable and converts into Phosphoric acid due to its strong reducing properties.
Phosphorous acid appears as colorless and odorless crystalline solid. The melting point of Phosphorous acid is 70.1∘C or a solution of the solid, its boiling point is 200∘C and its density is 1.651 cm3g . The contact with Phosphorous acid may severely irritate skin, lips, eyes and mucous membrane which may lead to nausea, vomiting and cramps in the stomach and may result in permanent damage. Phosphoric acid is used in dental cements, as a raw material for synthetic fibers and in the sugar industries.
Note: H3PO3 is a conjugate acid of a dihydrogen phosphite. It is a tautomer of phosphonic acid. It has strong reducing properties and tends to be converted into Phosphoric acid. Phosphorous acid is stronger than Phosphoric acid (H3PO3) .
