Question
Question: What is the name of above reaction 
(A) Hoffman rearrangement.
(B) Benzilic- acid rearrangement.
(C) Baeyer Villiger oxidation.
(D) Iodoform reaction.
Solution
The reactant in the given reaction is a diketone since it has two ketone groups, i.e. RCOR. Given that it is treated initially with a base, i.e. NaOH and then with an acidic proton, H+. Using these reagents, we can determine the type and name of reaction. We should be knowing the reagents used in any of the named reactions and substrates on which they are used as well. If we remember the substrate and reagent of the name reactions then we can classify as well as identify them.
Complete step by step answer:
Hoffman rearrangement is the reaction in which the reactant is primary amide and it is converted to primary amine with the help of reagents KOH/NaOH+Br2 . It is also called Hoffman degradation sometimes.
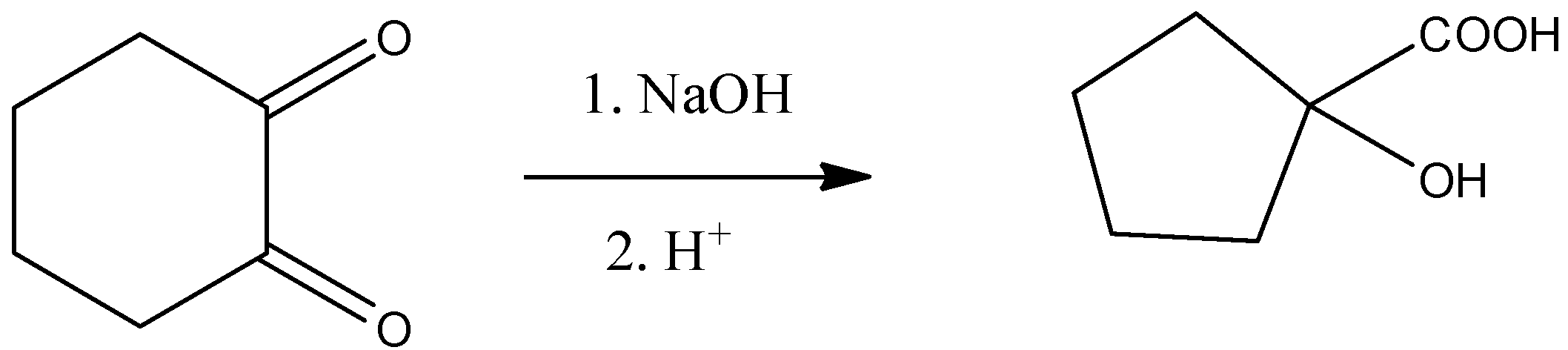
In Baeyer Villiger oxidation is the addition of oxygen in the substrate used. In this type of oxidation, the reagents are peroxy-acids or peroxides and a ketone is converted to ester by addition of nascent oxygen and cyclic ketones get converted into lactones.
Iodoform reaction is the test of a methyl ketone. In this reaction the reagents used are iodine and sodium hydroxide and they oxidize the methyl ketone to carboxylate. The reaction produces CH3I which is a pale-yellow color precipitate called iodoform which is a characteristic of this test.
Benzylic rearrangements have diketones as the substrate. It is a rearrangement of diketone. The reagents used is a base like KOH which turns benzene into benzoic acid. Below is the mechanism of the reaction.
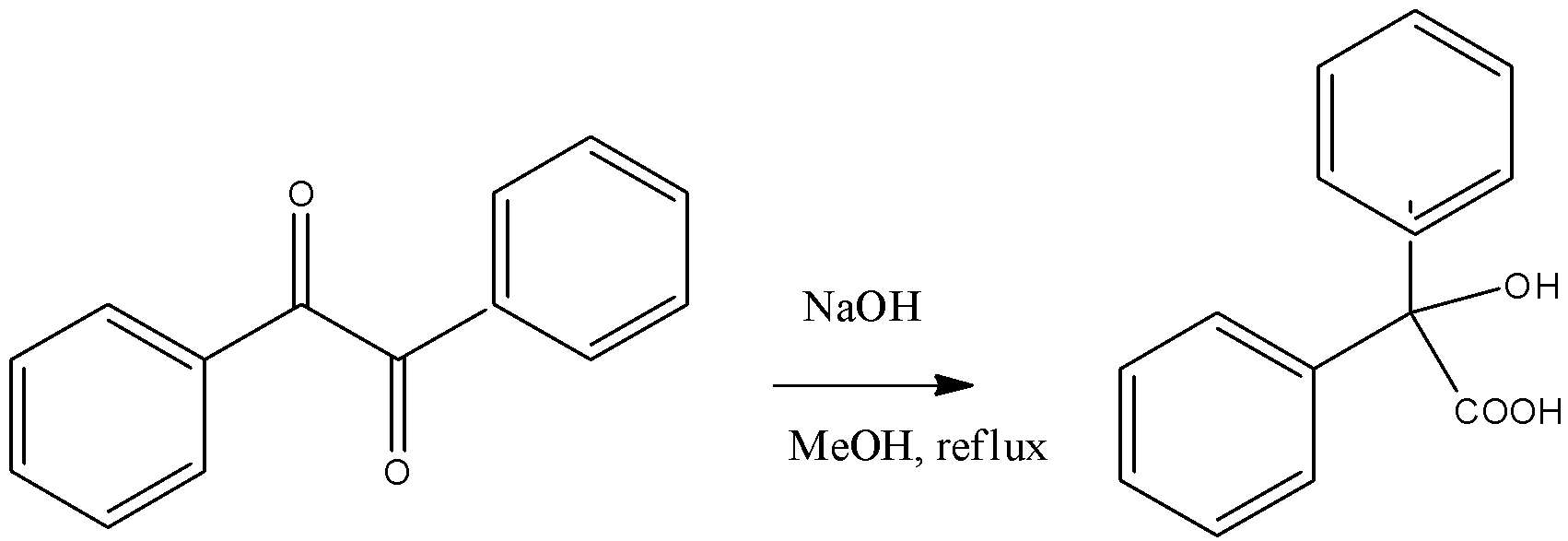
The substrate in the question is a diketone so it is Benzylic rearrangements
So, the correct answer is Option A.
Note: In this reaction the hydroxide attacks on one the ketone and when the lone pair of oxygen comes down the substituent attached to that ketone migrates to the other ketone carbon making an alcohol functional group there.
In the first step only, the base is used and a carboxylate salt of that metal is formed and when in the next step we hydrolyze the product then the metal is replaced by hydrogen and alcohol is formed. The presence of alcohol and carboxylic acid on the same carbon is called benzoic acid.
