Question
Question: What is the molecular geometry of the \(C{S_2}\) molecule?...
What is the molecular geometry of the CS2 molecule?
Solution
We have to know that the molecular geometry of a molecule could be predicted using VSEPR theory. With the help of VSEPR theory, we could determine the steric number. We can define steric number as the sum of the total bond pairs linked to the central atom and total lone pairs present in the central atom.
Complete answer:
In the hint part, we saw the VSEPR theory is used to determine the geometry of the molecule with the help of the number of electron pairs enclosing the central atom of the molecule. Based on this theory, the Lewis structure of the molecule has to be drawn and the steric number has to be determined to determine the geometry.
We can draw the Lewis structure of CS2 as,
The number of valence electrons in carbon=1×4=4 electrons
The number of valence electrons in sulfur=2×6=12 electrons
The total number of valence electrons in CS2 is sixteen electrons.
The Lewis structure of CS2 is,
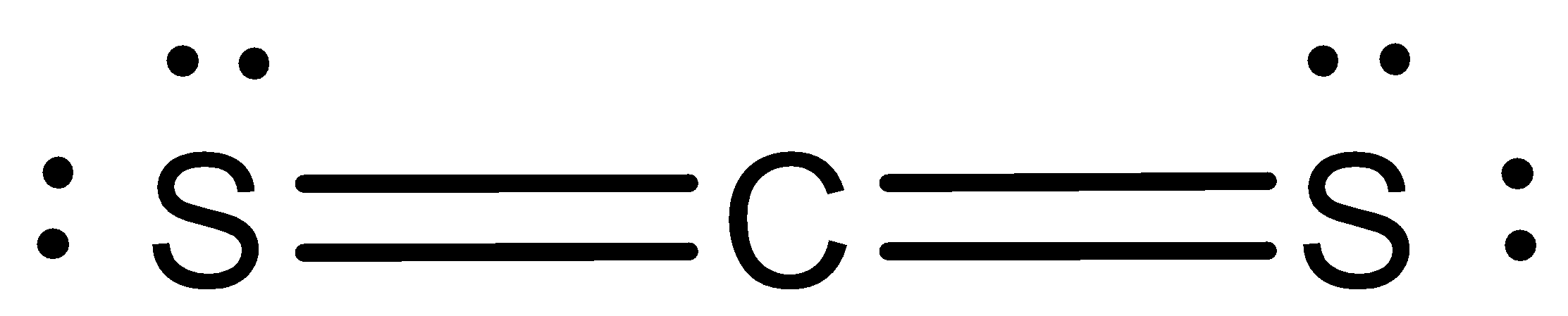
Let us now predict the steric number. We can write the formula of steric number as,
Steric Number=No. of bonded pairsincentral atom+Number Lone pairs present in central atom From the Lewis structure, let us now calculate the steric number. We can see that two atoms of sulfur are bonded to the central carbon atom, so the number of bonded pairs in the central atom is two. In carbon, no lone pairs are present.
So, the steric number is calculated as,
Steric Number=No. of bonded pairsincentral atom+Number of lone pairs present in central atom Stericnumber = 2 + 0
Stericnumber = 2
So, the steric number is two. For steric number two with zero lone pairs on the central atom, the electron pair arrangement is linear and molecular geometry is also linear.
The molecular geometry of the CS2 molecule is linear.
Note:
We have to know that if there is a lone pair present in the central atom, then the electron pair arrangement and molecular geometry would be different. If there is no lone pair present in the central atom, then the electron pair arrangement and molecular geometry would be the same.
