Question
Question: What is the molecular geometry of \[S{O_3}^{2 - }\]?...
What is the molecular geometry of SO32−?
Solution
We have to remember that a sulfate ion, SO32− has three oxygen atoms bonded to the sulfur atom, plus one lone pair on the sulfur atom. This gives four regions of electron density total, which gives a tetrahedral electron arrangement but we need to check the placement of atoms.
Complete Answer:
The molecular structure of SO32− can be represented as:
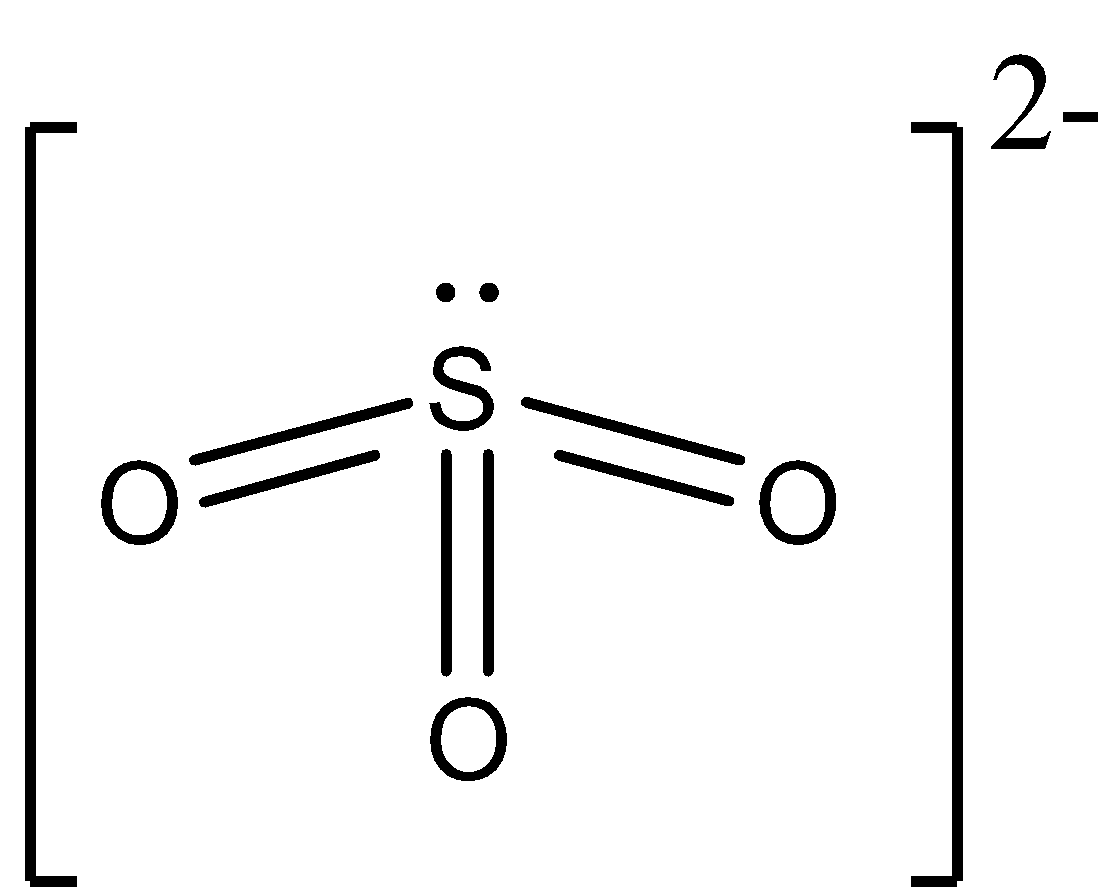
The central atom is sulfur contributing six electrons while oxygen contributes two electrons since three oxygen atoms are present thus six electrons will be contributed and two electrons for the negative charge which makes a total of fourteen valence electrons. Thus the total VSEP is seven. As we can see in the structure there are three 3 bonding pairs, the structure is trigonal pyramidal, with 109.5 bond angles. The Lewis Dot Structure for SO32− . The sulfite anion (SO32− ) is present in wines, and is used as preservative in certain foods. The accepted Lewis structure predicts both single and double bonds between S and O atoms involving available valence electrons. The Molecular structure and bonding Gaseous SO3 is a trigonal planar molecule of D3h symmetry, as predicted by VSEPR theory. SO3 belongs to the D3h point group. In terms of electron-counting formalism, the sulfur atom has an oxidation state of plus six and a formal charge of zero. The Lewis structure consists of an S=O.
Note:
We need to remember that the sulfite is a sulfur oxoanion that is the conjugate base of hydrogen sulfite (H2SO3).It is a sulfur oxoanion, a sulfur oxide and a divalent inorganic anion. It is a conjugate base of a hydrogen sulfite. Sulfite ion is one of the oxyanions of sulfur. Sulfur is at plus four oxidation states in SO32−.
