Question
Question: What is the molecular geometry of \( S{{I}_{6}} \) ? Draw its VSEPR and Lewis structure....
What is the molecular geometry of SI6 ? Draw its VSEPR and Lewis structure.
Solution
Hint : We know that for solving the given problem, the knowledge of VSEPR and Lewis structure should be present. Lewis structure helps in representing valence electrons of a molecule. On calculating, the formal charges with respect to the Lewis structure, the charge on a polyatomic ion can be defined easily. VSEPR theory is the valence shell electron pair repulsion theory. It helps in the prediction of the geometrical structure of the compound.
Complete Step By Step Answer:
Let's first try to draw the Lewis structure of trichloride. It has three chlorine atoms and only one boron so boron will be a central atom in this molecular structure. If the remainder is 1 , then l.p=1 and if the remainder is 2 and greater than 2 , then it should be divided by 2 to get the quotient as a lone pair.
Step-1: The molecular geometry of the compound. is identified using VSEPR theory. The rules for VSEPR are given :
Simple Lewis structure is obtained.
Bonds between 2 atoms are always considered 1 .
Here, geometry is total ; Electron pair = bond pair + lone pair
Step-2: By adding the total number of valence electrons, we get 6 valence electron of Sulphur and 7 valence electron of Iodine. So, total valence electrons =6+7×6=48 valence electron.
Step-3; By dividing total valence electrons by 8 , we get the bond pair as quotient.
So, 48÷8=6 bond pair.
Since, there is no remainder, so, l.p=0 .
Step-4: When e.p=l.p+b.p=6 , the shape obtained is octahedral.
The Lewis structure of SI6 is given as follows :
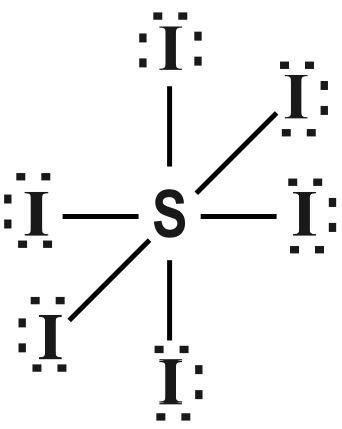
Note :
Remember that VSEPR along with geometrical structure also determines the shape of the molecule. The electrons in VSEPR occupy the positions that make the repulsion less. : VSEPR theory is basically used to predict the shape of molecules by using systematic steps. Whereas, lewis structure is also known as lewis dots, it is a representation of valence electrons in a given molecule.
