Question
Question: What is the molecular geometry of \( {H_2}O \) ? Draw its VSEPR structure....
What is the molecular geometry of H2O ? Draw its VSEPR structure.
Solution
VSEPR theory basically shows the molecular shapes, the full form is valence shell electron pair repulsion. The basic steps are find the central atom then count its valence electrons after that add one electron for each bonding atom then add or subtract electrons for charge at the end divide it by two to find total number of electron pairs and this number will help us to predict the geometry of the shape.
Complete answer:
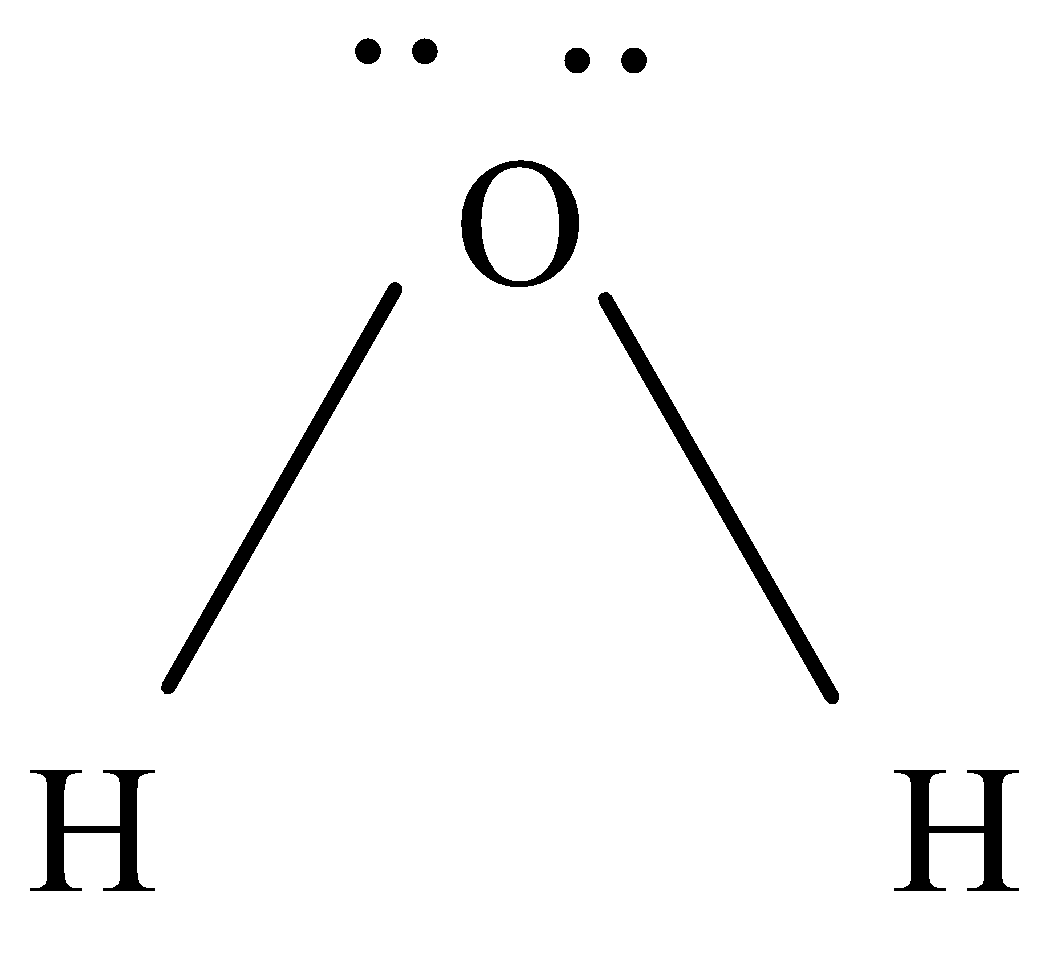
To know the molecular geometry we will draw lewis structure.
As we know we have two hydrogen ions and one oxygen, oxygen will be at the centre.
So, the central bond has two bonds with hydrogen and two lone pairs.
They are arranged in tetrahedral shape. The angle of H−O−H is 104.5o
So basically, water has bent geometry or V-shaped geometry due to the presence of lone pairs. The hybridization of water is sp3 .
Note:
The V-shape either occurs due to lone pair-lone pair, lone pair-bond pair or bond pair- bond pair repulsion. Understanding the molecular structure of any compound helps us to understand polarity, reactivity, magnetism and many other properties.
