Question
Question: What is the molecular geometry of \( BC{l_3} \) ? Draw its VSEPR and Lewis structure....
What is the molecular geometry of BCl3 ? Draw its VSEPR and Lewis structure.
Solution
VSEPR theory is basically used to predict the shape of molecules by using systematic steps. Whereas, lewis structure is also known as lewis dots, it is a representation of valence electrons in a given molecule.
Complete answer:
Let's first try to draw the Lewis structure of trichloride. It has three chlorine atoms and only one boron so boron will be a central atom in this molecular structure.
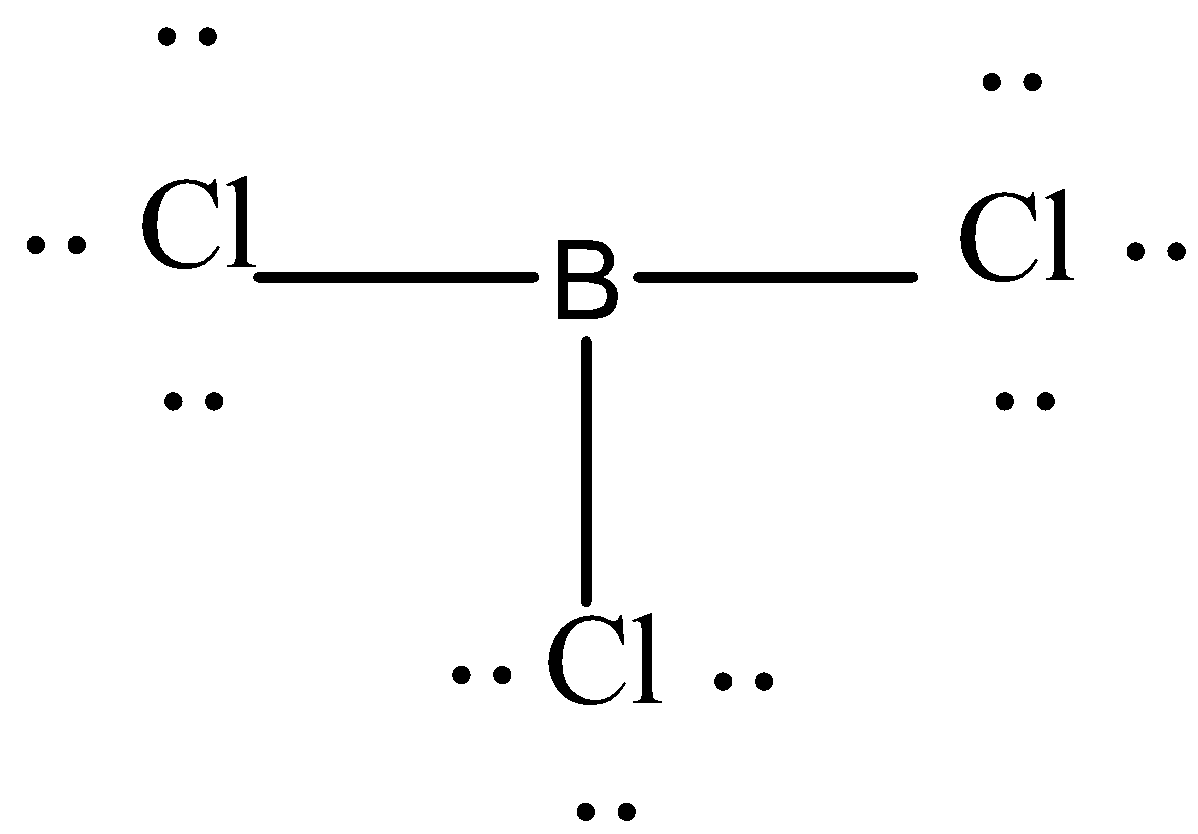
In this Lewis structure every chlorine has three lone pairs of electrons around it.
That is why it has sp2 hybridisation so the molecular geometry will be trigonal planar and the bond angle will be 120o .
So the VSEPR structure will look something like this:
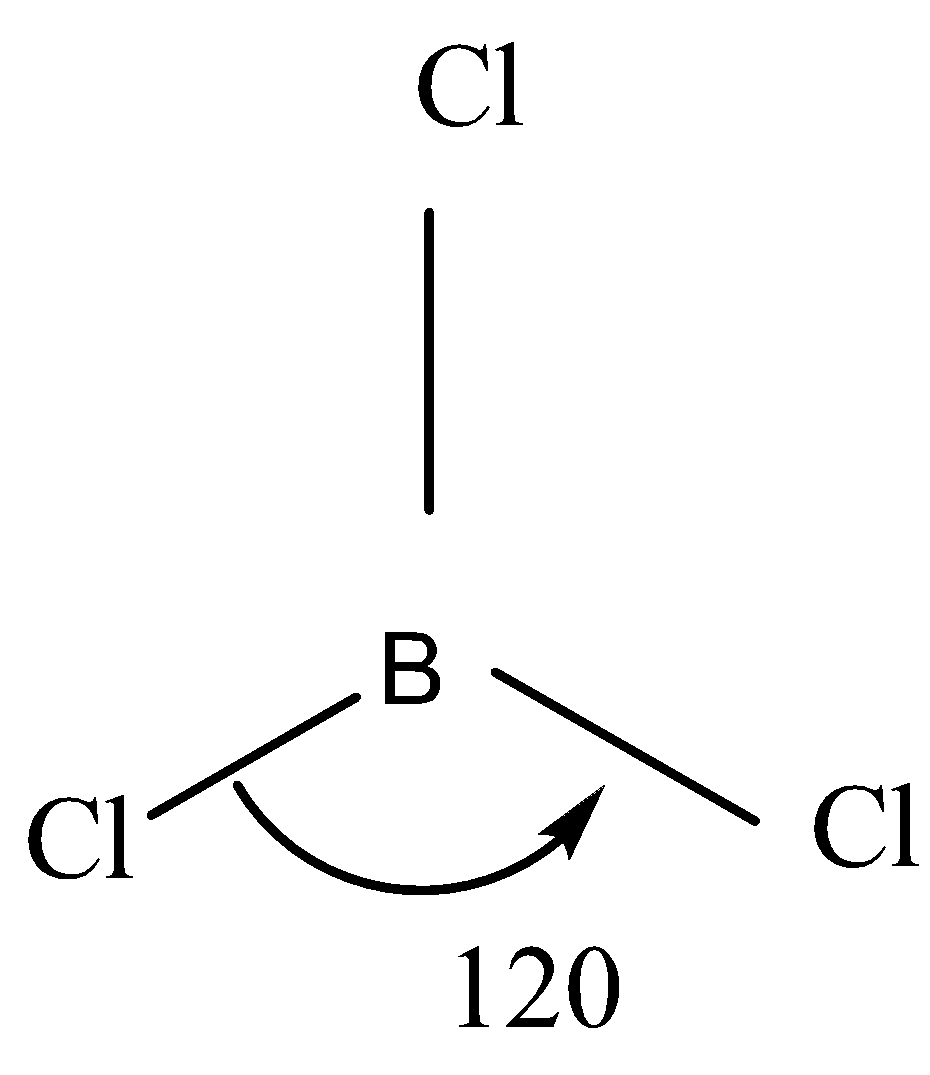
Note:
VSEPR is valence shell electron pair repulsion, it simply tells us that nonbonding and bonding electron pairs of the central atom in a molecule push each other away from each other in 3D space due to which molecules acquire their individual molecular shapes.
