Question
Question: What is the molecular formula of Strontium Chloride? A) \[SnC{l_2}\] B) \[SrC{l_2}\] C) \[PbC{...
What is the molecular formula of Strontium Chloride?
A) SnCl2
B) SrCl2
C) PbCl2
D) None of the above
Solution
The strontium is a divalent atom having an ionic symbol Sr2+. We need to know that Sr has +2 valency whereas chloride has −1 valency therefore, the formula of strontium chloride turns out to be SrCl2.
Complete step by step answer:
We need to know that the strontium chloride is an inorganic salt containing strontium and chlorine atoms. The salt so formed has no charge on it making it a neutral salt. We also need to know that the Sr is an alkaline earth metal having atomic number 38 and we also know that it has its properties intermediate between Ba and Ca.
Electronic configuration of Sr: [Kr]5s2
Now we discuss about the preparation of strontium chloride as,
SrCl2can be prepared by using strontium hydroxide and hydrochloric acid:
Sr(OH)2+2HCl→SrCl2+2H2 We can be draw the structure of SrCl2 can be represented as:
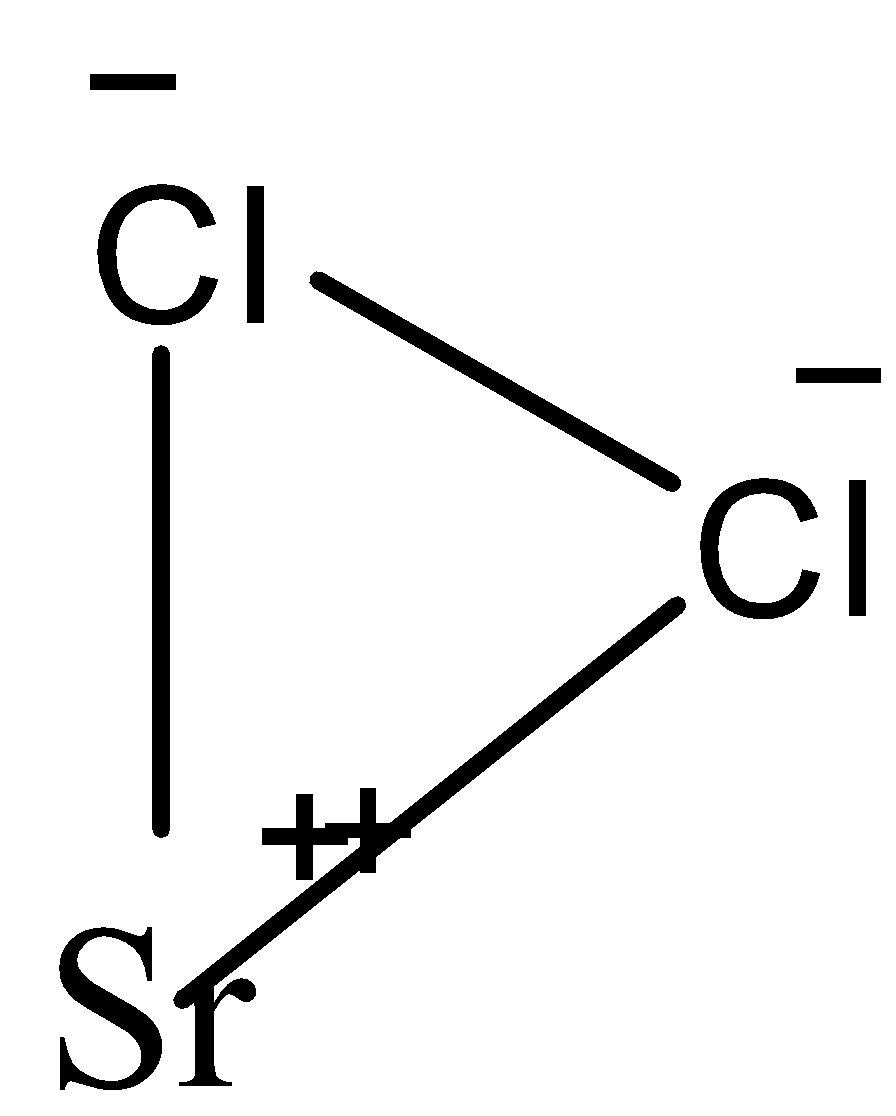
Let's look at the given options:
Option A) this is an incorrect option as SnCl2 this is tin chloride.
Sn is an atomic symbol of Tin.
Option B) This is a correct option as SrCl2 is a correct molecular formula for strontium chloride as explained above.
Option C) this is an incorrect option as PbCl2 this is lead chloride.
Pb is an atomic symbol of lead.
Option D) this is an incorrect option as we got a correct option as option A.
Hence, the correct answer is, ‘Option A’.
Note: The strontium chloride has a molecular weight of 158.53g/mol . We have to know that it is white crystalline powder and an odorless salt. The solubility of SrCl2is in water and little in alcohol.
