Question
Question: What is the mechanism of an Aldol addition?...
What is the mechanism of an Aldol addition?
Solution
To solve this question we should know about:
Aldol reaction: Aldehyde and alcohol are abbreviated as 'Aldol.' The Aldol Reaction occurs when the enolate of an aldehyde or ketone combines with the carbonyl of another molecule at the -carbon under basic or acidic circumstances to produce -hydroxyl aldehyde or ketone.
So, we draw diagrams from hybridization. We try to understand the nature of functional groups.
Complete Step By Step Answer:
Under basic or acidic circumstances, an enolate of an aldehyde or ketone is added to the carbonyl carbon of another molecule to produce a -hydroxyaldehyde or -hydroxyketone (an aldol).
The base-catalyzed reaction of acetaldehyde with itself to create 3-hydroxybutanal is an example of aldol addition.
The aldol addition is broken down into three phases.
-The formation of a resonance-stabilized enolate ion by the removal of an acidic -hydrogen by a strong base.
Base catalyzed
-The enolate ion generates a new C−C bond and an alkoxide ion by nucleophilically attacking the electrophilic C=O group of another molecule. Aldol is formed by protonation of the alkoxide ion.
-Heating the aldol with a strong base results in the elimination of water and the formation of an α,β− unsaturated product.
All the above steps can be explained by the given reactions
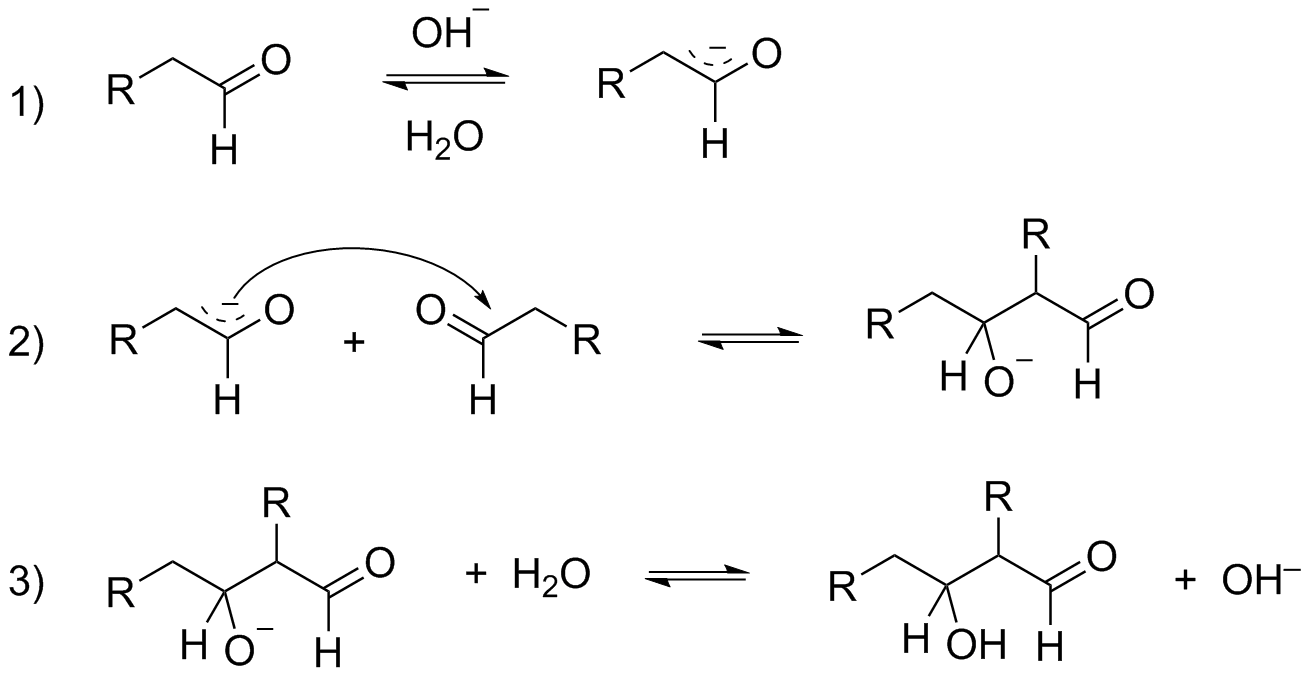
A condensation reaction occurs when two molecules combine to form a larger molecule while a smaller molecule, such as water, is lost.
The overall process is called an aldol condensation if the aldol is then dehydrated.
Note:
The initial step in the reaction mechanism involves acid-catalyzed tautomerization of the carbonyl molecule to the enol when an acid catalyst is used. By protonation, the acid also acts to activate the carbonyl group of another molecule, making it highly electrophilic. Because the enol is nucleophilic at the -carbon, it can attack the protonated carbonyl molecule, resulting in the aldol following deprotonation. The unsaturated carbonyl molecule is formed when this dehydrates. The diagram depicts a typical acid-catalyzed aldehyde self-condensation.
