Question
Question: What is the maximum number of layers of atoms in close-packed planes that will lie within two imagin...
What is the maximum number of layers of atoms in close-packed planes that will lie within two imaginary parallel planes having a distance between them as 1332r (r is the radius of an atom) in the copper crystal (fcc)?
[ Consider the atoms to be within the parallel planes if their centres are on or within the two parallel planes.]
Solution
Unit cells are basically the smallest repeating atoms in a crystal. There are different types of unit cell like body centred unit cell represented as BCC, face centred unit cell (FCC). FCC is the face centred cubic unit cell, which is having coordination number 12 and also contains 4 atoms per unit cell.
Complete step by step answer:
- We will first draw the diagram:
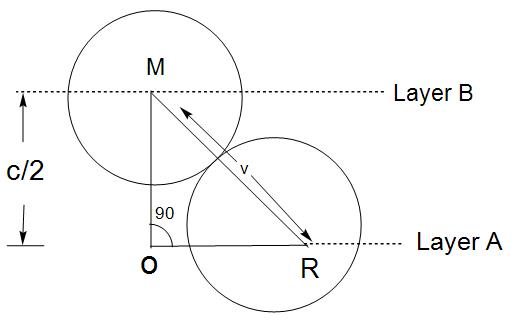
- Here, we know that the distance given in between parallel planes is 2C 2C$$\begin{aligned}
& \left( OM \right)+\left( RO \right)={{\left( MR \right)}^{2}} \\
& \left( \frac{C}{2} \right)+\left( \frac{2r}{\sqrt{3}} \right)={{\left( 2r \right)}^{2}} \\
\end{aligned}$$
- Now, by solving this equation we get :
