Question
Question: What is the maximum number of grams of \[N{H_4}Cl\] that will dissolve in \[200\] grams of water at ...
What is the maximum number of grams of NH4Cl that will dissolve in 200 grams of water at 70 degree Celsius?
Solution
Remember that to dissolve is to enable a solute to completely enter into a solution; this is generally used in Chemistry. Dissolution is another word for dissolving. Dissolution usually means for the solid form to complete transition into a liquid form. Take the help of a solubility graph to further solve the question.
Complete answer:
Let us note down the given values;
The compound given to us is ammonium chloride that is NH4Cl.
The mass of water is given as 200 grams.
The temperature at which the process is to be carried out is 70 degree Celsius.
So what we need to do is find the maximum mass of ammonium chloride that can dissolve at the given values of temperature and mass of water. This means the mass that is completely soluble must be found.
We must take the help of a solubility graph in order to clearly recognize how much of a given salt dissolves at different temperatures. The graph we are using have the x-axis as the ‘temperature at degree Celsius’ and the y-axis gives ‘solute per 100 grams of water’.
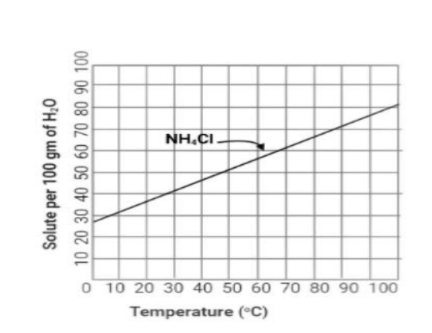
Now since we are given the temperature at which the dissolution takes place, that is 70 degree Celsius, so we look at the x-axis of the graph where 70 degrees has been marked. Look at the y-axis for the value corresponding to 70 degrees on the x-axis. The y-axis value we find is approximately 62 grams.
So we can infer in general that 62 grams of solute per 100 grams of water gets dissolved at 70 degrees Celsius. This also means that at 70 degree Celsius, no more than 62 grams can be dissolved in 100 grams of water. Any mass of salt added after 62 grams will not be soluble in the water.
Now that we know the solubility of ammonium chloride in 100 grams of water, we are asked to find solubility in 200 grams. This can be found using unitary method;
If 100 grams of water dissolves 62 grams ⇒100g→62g
⇒1 gram of water would dissolve =10062 grams of ammonium chloride
Then 200 grams of water would dissolve =?
⇒Number of grams of ammonium chloride dissolved in 200 grams of water =200×10062
∴Number of grams of ammonium chloride dissolved in 200 grams of water =124g of NH4Cl.
Note:
The dissolution of solute per 100 grams of water for each salt varies. To find the corresponding values of solubility, each salt can be plotted on the solubility curve, from there we can easily find the level of solubility of each salt at any given temperature.
