Question
Question: What is the mass of three moles of \[{C_2}{H_2}\]?...
What is the mass of three moles of C2H2?
Solution
We have to remember that the acetylene is a simplest alkyne and it is a hydrocarbon having the formula C2H2. And this compound is also known as ethyne. Acetylene is a colorless gas and it is used as fuel.
Complete answer:
We also know that a mole is used to specify the amount of material and it contains 6.022×1023 particles. Hence, one mole is equal to 6.022×1023 particles and this number is called Avogadro number. The mole is mainly used to express the number of products and the reactants. Hence, it is a count of particles. The mass of one mole of substance is equal to the molar mass of the compound. Therefore, the number of moles is equal to the amount of samples. Acetylene is a simplest alkyne and it is a hydrocarbon having the formula C2H2. We must remember that acetylene is a compound with two carbon atoms and two hydrogen atoms. The mass of three mole of acetylene is equal to its molecular mass.
The molar mass of carbon, C=12
The molar mass of hydrogen, H=1
To find the mass of acetylene, add the molar mass of elements. Therefore,
The mass of three mole of acetylene =2×12+2×1=26
Hence the mass of three mole of acetylene is equal to 26g.
The structure of acetylene can be drawn as,
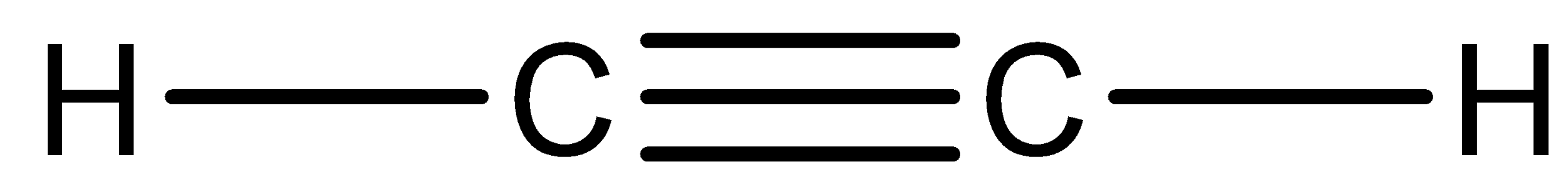
And this acetylene is mainly used in the fabrication industry and it is used as fuel for welding. And it is a highly unstable compound. It may lead to explosion under high pressure or high temperature conditions.
Note:
We must have to remember that acetylene is the simplest alkyne having the formula, C2H2. And it has two carbon atoms and two hydrogen atoms. The mass of a compound is equal to its molecular mass. Hence, the mass of three mole of acetylene is equal to 26g.
