Question
Question: What is the mass of oxalic acid, \({{H}_{2}}{{C}_{2}}{{O}_{4}}\) , which can be oxidised to\(C{{O}_{...
What is the mass of oxalic acid, H2C2O4 , which can be oxidised toCO2 by 100ml of MnO4−solution ,10 ml of which is capable of oxidising 50 ml of 1.00 N I− toI2 ?
A. 2.25g
B. 52.2g
C. 25.2g
D. 22.5g
Solution
Oxalic acid is having the formula H2C2O4.It is also called as ‘’crab acid’’, it is actually a white crystalline solid which is generally found to get dissolved in solution and become colourless. Molecular formula of oxalic acid in dehydrate form is:
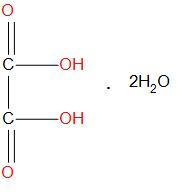
The formula used to calculate the weight for oxalic acid is:
N1×V1=molar massweight×valency factor×1000
Where, N1is the normality of MnO4− and V1 is the volume of MnO4−
Complete answer:
-We are given solution that is 100 ml MnO4−, then it is asked that if we add 10ml in 50ml of 10N I−, then it will be totally converted into I2, so we can write its equation as :
