Question
Question: What is the mass of one mole of barium acetate, \[Ba{\left( {{C_2}{H_3}{O_2}} \right)_2}\]?...
What is the mass of one mole of barium acetate, Ba(C2H3O2)2?
Solution
We need to remember that the barium acetate is a chemical compound having the formula, Ba(C2H3O2)2. It is a salt of barium and acetic acid. And the barium acetate is mainly used for manufacturing and it is used for the preparation of other acetates.
Complete answer:
We also need to know that a mole is used to specify the amount of material and it contains 6.022×1023 particles. Hence, one mole is equal to 6.022×1023 particles and this number is called Avogadro number. The mole is mainly used to express the number of products and the reactants. Hence, it is a count of particles. The mass of one mole of substance is equal to the molar mass of the compound. Therefore, the number of moles is equal to the amount of samples.
As we know that the barium acetate is a chemical compound having the formula, Ba(C2H3O2)2. It is a salt of barium and acetic acid. And the barium acetate is mainly used for manufacturing and it is used for the preparation of other acetates. The mass of one mole of barium acetate is equal to its molecular mass.
The molar mass of barium, Ba =137
The molar mass of carbon, C=12
The molar mass of hydrogen, H=1
The molar mass of oxygen, O=16
To find the mass of barium sulphate, add the molar mass of elements. Therefore,
The mass of one mole of barium acetate =137+4×12+6+4×16
=255
Hence, the mass of one mole barium acetate is equal to 255. Barium acetate is a strong electrolyte and it does not undergo hydrolysis. And the structure of barium acetate can be drawn as,
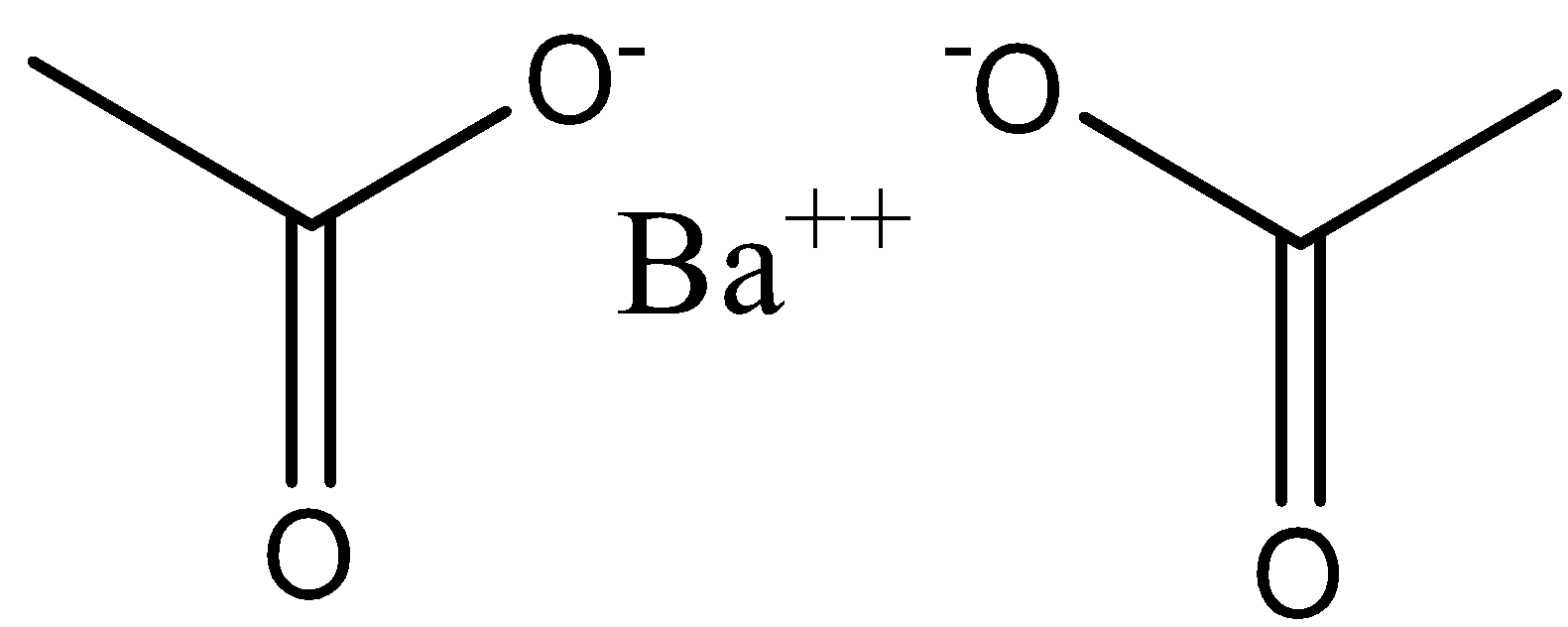
Note:
As we know that the mass of one mole of barium acetate is equal to its molecular mass. Hence, the mass of one mole of barium acetate is equal to 255. The barium sulphate is used as a catalyst in organic synthesis. And the barium acetate is prepared by the reaction of acetic acid with barium carbonate. And the reaction can be written as,
BaCO3+2CH3COOH→(CH3COO)2Ba+CO2+H2O
