Question
Question: What is the major product of the following reaction? 
(A)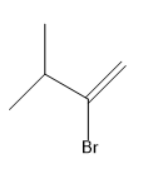
(B) 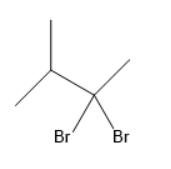
(C) 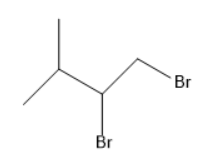
(D) 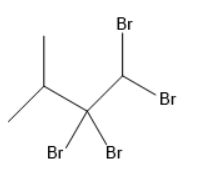
Solution
Alkynes are unsaturated hydrocarbons consisting of a triple bond between one or more carbon and carbon atoms. When alkynes are treated with excess Hydrobromic acid (HBr) the bromine atom attacks on the unsaturated carbon with more carbon substitution and forms bromo alkanes.
Complete answer:
Given molecule is an alkyne which has the unsaturation or triple bond at 1st position. The IUPAC nomenclature of the given molecule is 1 -isopentane.
When this compound is treated with excess Hydrobromic acid (HBr) the addition of this reagent takes place according to Markovnicov rule. This rule states that the negative part of the reagent attacks on the carbon containing more carbon substitution and the positive part of the reagent attacks on the carbon containing less carbon substitution.
The negative part of the reagent is bromide ion and the positive part of the reagent is a proton.
The addition of Hydrobromic acid (HBr) to the given molecule takes place as follows:
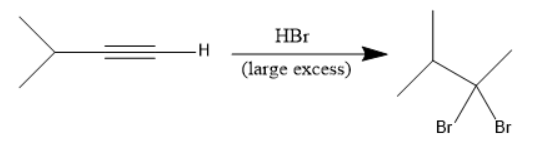
In the above reaction, at first the bromine attacks on the internal unsaturated carbon forms an alkene. After that another mole of Hydrobromic acid (HBr) attacks to form a geminal dibromide.
In the given options, geminal dihalide is in option B.
Option B is the correct one.
Note:
In presence of peroxides, the addition of a reagent to unsaturated hydrocarbons takes place according to anti-markovnikov rule. As in the above reaction there is no presence of peroxides thus the reaction takes place according to the Markovnikov rule.
