Question
Question: What is the major product formed in the following reaction? 
Answer is, there is Br in place of OH. Why is there no Hydride shift?
Solution
In the given reaction, we can see 1−methoxy−2−methylpropan−1−ol reacts with HBr . The product of the above reaction is 1−bromo−1−methoxy−2−methylpropane.
Complete answer:
We have to know the structure of the reaction between 1−methoxy−2−methylpropan−1−ol and HBr has to be drawn.
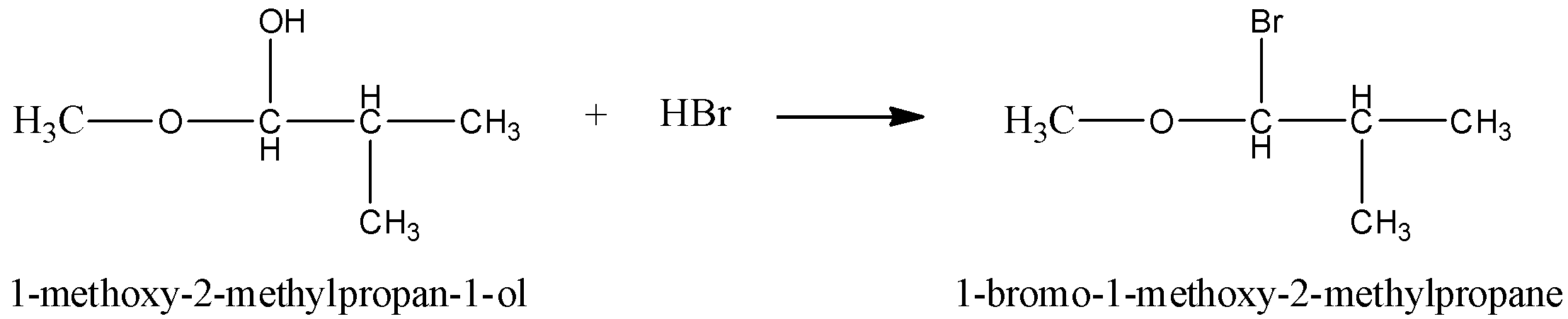
Then, we have to see the mechanism for the reaction between1−methoxy−2−methylpropan−1−olreacts with HBr has to be drawn.
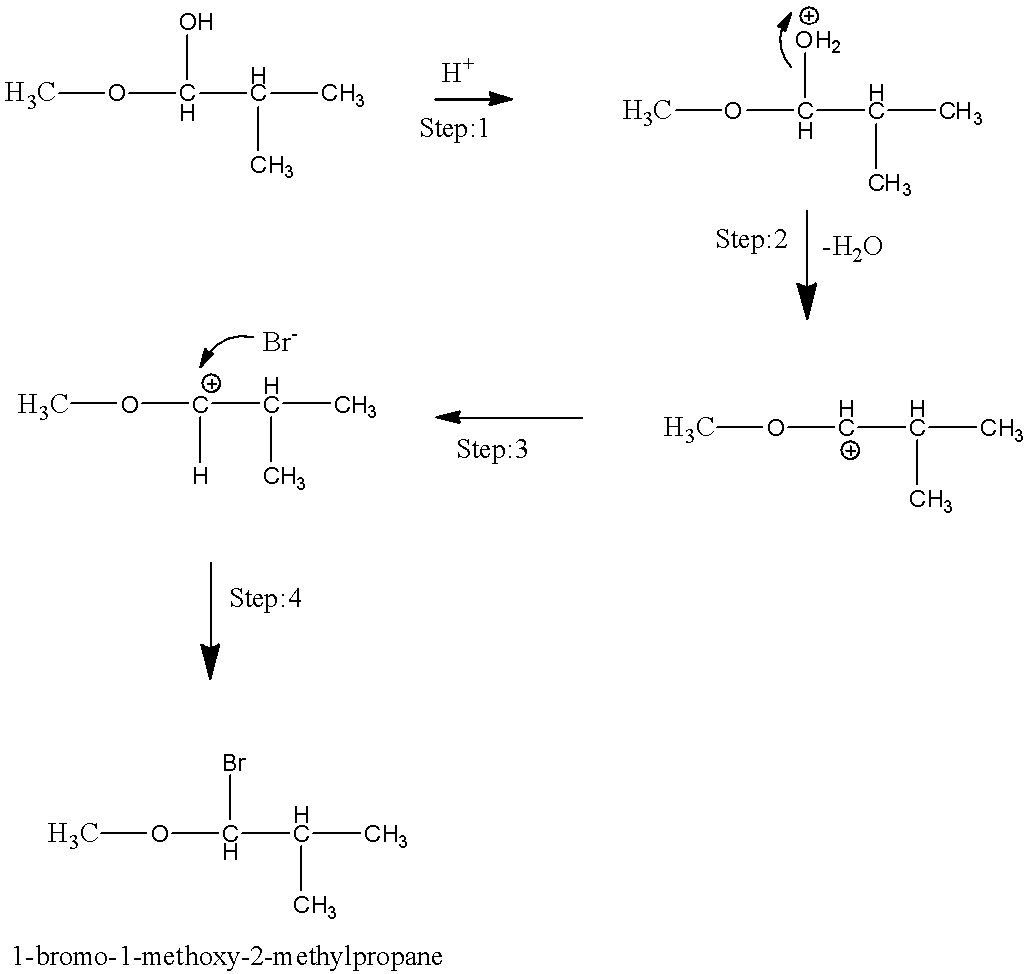
We have to know the above mechanism. The step by step information of the above mechanism has to be given below. It has three main steps.
We have to know in the first step, −OH group on the first carbon has to be protonated. To form a water molecule.
We have to know in the second step, the formation of water molecules is lost, to form carbocation in the other step.
We have to know in the third step, the nucleophilic attack of bromide ion on the carbocation. It gives 1−bromo−1−methoxy−2−methylpropane.
The 1−bromo−1−methoxy−2−methylpropane is the final product of the given reaction.
In this above mechanism, no hydride shit formation is present. Because of the basic alkyl primary carbocations are too high in energy to shape so you don't in general see an essential carbocation. There are a few special cases for this overall standard for essential carbocations. On the off chance that a secondary carbocation is vicinal to tertiary carbon-bearing hydrogen, a 1,2−hydride shift ought to happen.
Note:
We have to remember that one revision pathway where an insecure carbocation can be changed into a more-steady carbocation is known as a hydride shift. Then, you can have various hydride shifts. This will restrict the quantity of hydride moves that can happen. You can likewise have carbocation movements to similarly subbed carbons and even moves to molecules farther away (like 1,4 shift).
