Question
Question: What is the major alkene formed in the following Hofmann elimination? 
A. i
B. ii
C. iii
D. iv
E. v
Solution
Hofmann elimination is the reaction where an aliphatic or aromatic amine that has beta-hydrogen is converted to an unsaturated compound. It takes place in the presence of a base along with high temperature (heat).
Complete answer:
Hofmann elimination is the reaction where an amine reacts with a base to form an alkene. The amine consist of a β - hydrogen whose removal marks the formation of a double bond. This elimination is based on the Saytzeff rule that states that the major product will be that product which is more stable.
The given compound when subjected to a base and heat, have the β - hydrogen removed, after the removal of β - hydrogen, the most stable carbocation will form the major product. The carbocation is stable when the saturation takes place on the 2∘ carbocation, which is the product (i), while other products contain less stable 1∘ carbocations. Therefore the reaction will be as follows:
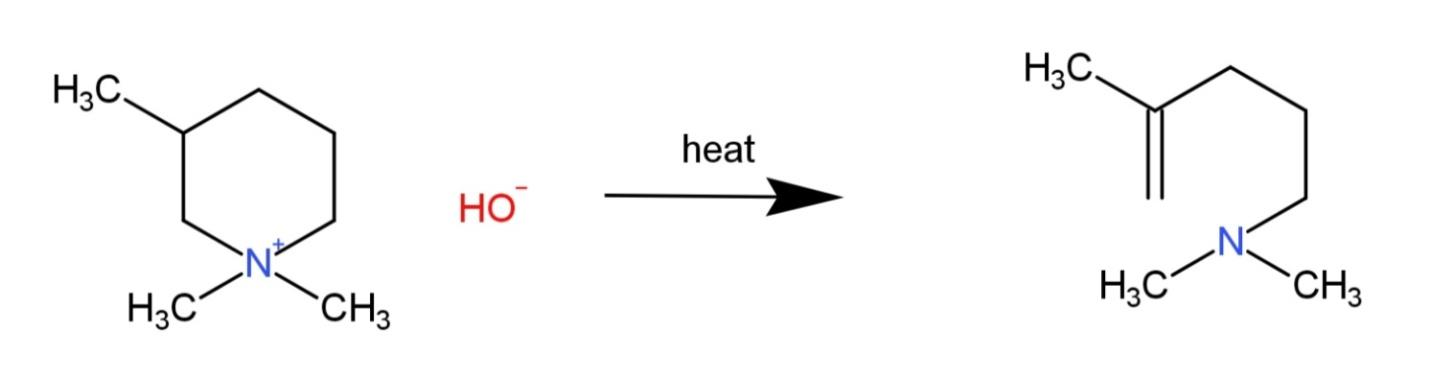
Hence, the product formed is (i), so, option A is correct.
Note:
Hofmann elimination reaction and Hofmann bromamide degradation reactions are two different reactions, but both consist of a base as a reactant. Hofmann bromamide consists of the formation of aliphatic or aromatic amines from the reaction of amide, bromine and sodium hydroxide (base). Hofmann elimination is the preparation of unsaturated compounds.
