Question
Question: What is the main factor responsible for weak acidic nature of \( {\text{B - F}} \) bonds in \( {\tex...
What is the main factor responsible for weak acidic nature of B - F bonds in BF3 ?:
(A) Large electronegativity of F
(B) Three centered two electron bonds in BF3
(C) pπ-dπ backbonding
(D) pπ-pπ backbonding
Solution
If a molecule has an atom with a lone pair of electrons and other atom has a vacant orbital adjacent to it, then back bonding occurs between the two atoms. The order of acidity of the boron trihalides can be explained on the basis of boron-halogen pi-backbonding.
Complete step by step solution:
BF3 is the formula of the inorganic compound boron trifluoride. It is a boron trihalide. In general, the boron trihalides can act as Lewis acids because they can very easily form adducts with electron pair donors (which are termed as Lewis bases). Thus, all the three boron trihalides which are boron trifluoride, boron trichloride and boron tribromide are capable of forming stable adducts with Lewis bases.
Since according to Lewis concept, a substance which accepts electron pairs are acids and substances which donate electron pairs are bases, thus the boron trihalides which possess a sextet of electrons accept an electron pair to complete its octet and thus behaves as Lewis acids.
Among the boron trihalides, boron trifluoride is the weakest Lewis acid. This is explained as follows. Each of the three boron trihalides have a planar trigonal geometry which arises because of the sp2 hybridization of boron atom in excited state. Now, one of the 2p orbitals remains vacant and unhybridized in sp2 hybridization and a halogen atom has a partially filled p orbital. Thus the partially filled halogen p orbital and the unhybridized orbital of boron will overlap and form a B - F sigma bond, whereas the rest of the three halogen orbitals will have one lone pair of electrons each. Now, the filled pz orbital of halogen atom will overlap laterally with the vacant boron pz orbital and will form an F→B π− bond which is called pπ−pπ backbonding.
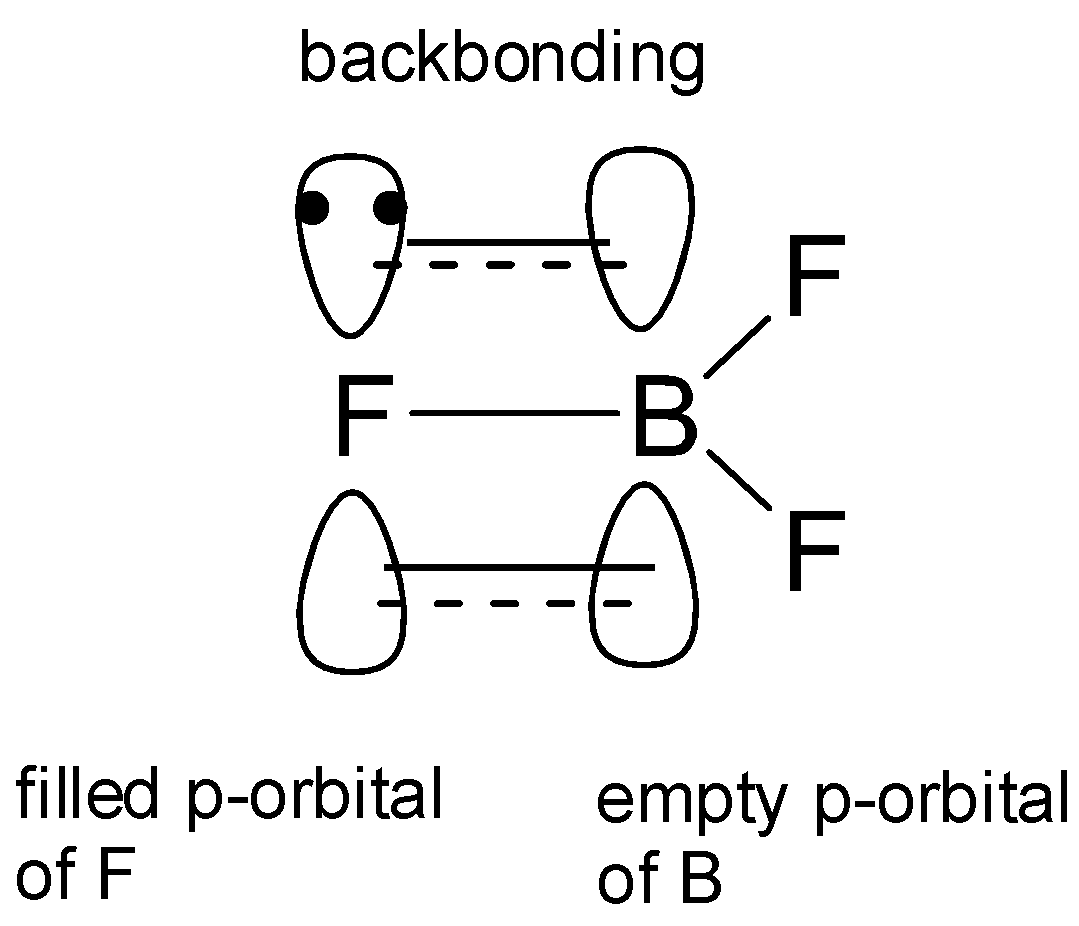
The overlap is most effective in BF3 as it involves orbitals of the same shape and energy. As we move from F to I, the effectiveness of this overlap reduces. Thus the tendency of pπ−pπ backbonding is maximum in BF3 and hence it will have minimum tendency to accept electron pairs and so it will be the least acidic.
So option D is correct.
Note:
The increase in the tendency of pπ−pπ backbonding by a molecule will lead to decrease in its tendency to accept electron pairs and thus decrease its Lewis acidity. Boron trifluoride is most commonly used as an organic synthesis reagent as a Lewis acid. For example, it is used for initiating the polymerisation reactions of polyethers. It is also used as a catalyst in some alkylation, dehydration, isomerization, condensation reactions. It is also used to prepare diborane.
