Question
Question: What is the main difference between atomic orbital and molecular orbital?...
What is the main difference between atomic orbital and molecular orbital?
Solution
Hint : The name itself suggests that the atomic orbitals are orbitals associated with individual atoms in their free state and the molecular orbitals are orbitals associated with molecules that contain two or more atoms in a bonded state.
Complete Step By Step Answer:
The orbitals according to Bohr’s atomic model or Schrodinger's model represent the three dimensional spaces around the nucleus where the probability of finding electrons is high. Mathematically, orbitals can be treated as wave functions that help us in doing various quantum mechanical calculations on them.
The atom in a free state has a uniformly distributed electron cloud around its nucleus resulting in its overall symmetry. When two or more atoms approach each other, their electron clouds are pulled by a force of attraction from each other’s nuclei. This pull results in the distortion of electron clouds disturbing the symmetry of the orbitals of the atoms.
When two atoms approach each other, their orbitals also come closer and the wave functions can experience a constructive or destructive overlap when the orbitals overlap to form a bond. The bonded electrons have a high electron density in between the two nuclei rather than a uniformly distributed electron cloud.
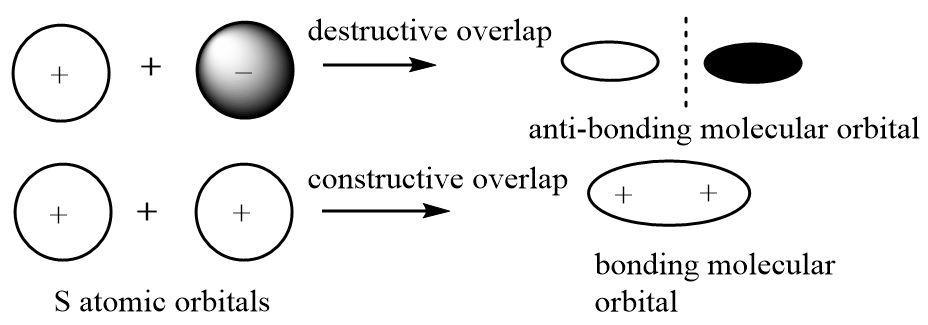
Thus atomic orbitals represent spaces with maximum electron density associated with individual atoms but molecular orbitals represent spaces of high electron density (bonding MO) between the nuclei of two atoms linked through a bond and also spaces where there is zero probability of finding shared pairs of electron in a molecule (non-bonding MO).
Note :
The overlap between two atomic orbitals can result in a variety of bonds. A head-on overlap between two orbitals results in the formation of a sigma σ bond between atoms and the sidewise overlap results in a π bond.
