Question
Question: What is the Lewis structure of \(N{O_2}\)?...
What is the Lewis structure of NO2?
Solution
Lewis’s structure is the pictorial representation of the valence electron present in an atom. It is the simplest way of presenting an electronic structure.
Complete step by step solution:
There are various ways of writing a Lewis structure. Lewis’s structure shows the arrangement of valence electrons around the molecules. Electrons are represented as dots and bonding pairs as lines.
Step 1:
For NO2 total number of valence electrons must be known.
N=5
O=6×2=16
Therefore total number of valence electrons are 16+5=17
We need to arrange this electron in such a way that the octet it fills.
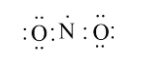
As we can see there are 5 electrons in nitrogen and 8 in oxygen.
Step 2:
Now we transfer a pair of electron from oxygen to make a double bond,
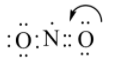
This way the final lewis structure looks like
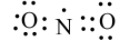
This is the lewis structure for NO2
Note:
One must write the best Lewis structure for an atom. The best structure is when the octet is complete with lowest formal charge. It does not explain the formation of the molecule or their geometry.
