Question
Question: What is the Lewis structure of \(N{O^ + }\)?...
What is the Lewis structure of NO+?
Solution
A simplified representation of a compound or a molecule that shows arrangement of bonded electrons i.e., the electrons which are participating to form a chemical bond, as well as number of non-bonded electrons i.e., number of lone electrons around an atom, is termed as its Lewis structure.
Complete answer:
To draw a Lewis structure of a compound or ion, we must stick to some important points which are as follows:
1.First, evaluate the sum of valence electrons of each atom and the charge (if present) which will be equal to the total number of valence electrons of the ion.
For the given ion NO+, the overall number of valence electrons are evaluated as follows:
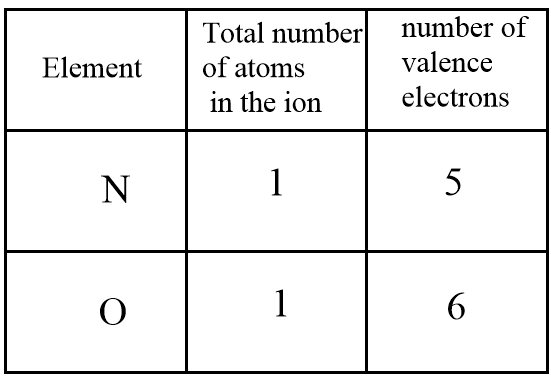
Also, in the molecule a positive charge is present i.e., an electron is donated by the atom. So, we have to subtract the number of electrons donated to calculate the total number of valence electrons in the ion.
Therefore, the total number of valence electrons in the given ion =5+6−1⇒10
2.Draw the basic structure of the ion in which all the atoms are connected via single bond:
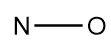
3.Now, add the required number of non-bonded electrons to each atom in the basic structure of the molecule:
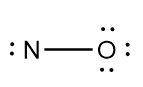
4.As the valency of each atom is incomplete in the structure, so we add multiple bonds i.e., double or triple bond as per requirement to complete the valency of each element in the molecule.
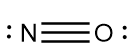
5.Calculate the formal charge of each atom in the molecule.
Formal charge of nitrogen atom =5−26−2⇒0
Formal charge of oxygen atom =6−26−2⇒+1
Hence, the nitrogen atom has a zero formal charge but the oxygen atom has a formal charge of +1 in the molecule.
6.The formal charge is allocated on the atom in the structure.
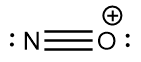
Hence, the Lewis structure of nitrosonium ion i.e., NO+ is as follows:
Note:
A theoretical charge assigned to the elements of molecules assuming that the electrons participating in the formation of bonds are shared equally between the atoms. The best Lewis structure is considered in which the formal charge is zero or near to zero. The formula to calculate formal charge is as follows:
FC=V−2B−L
Where, V is the number of valence electrons, B is the number of electrons participating in the formation of bonds and L is the number of line pairs of electrons on an atom.
