Question
Question: What is the Lewis structure of \[N{H_3}\]?...
What is the Lewis structure of NH3?
Solution
We need to know that the ammonia is a sp3 hybridized molecule having trigonal pyramidal geometry. The three hydrogen atoms are attached to the centrally present atom that is nitrogen. Ammonia is a gas having molecular mass 17g/mol. It is a colorless alkaline gas.
Complete answer:
We need to know that the Lewis structure of ammonia NH3, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons on top of the atom. This is the reason why ammonia acts as a Lewis base, as it can donate those electrons.
Lewis structure of ammonia can be represented as,
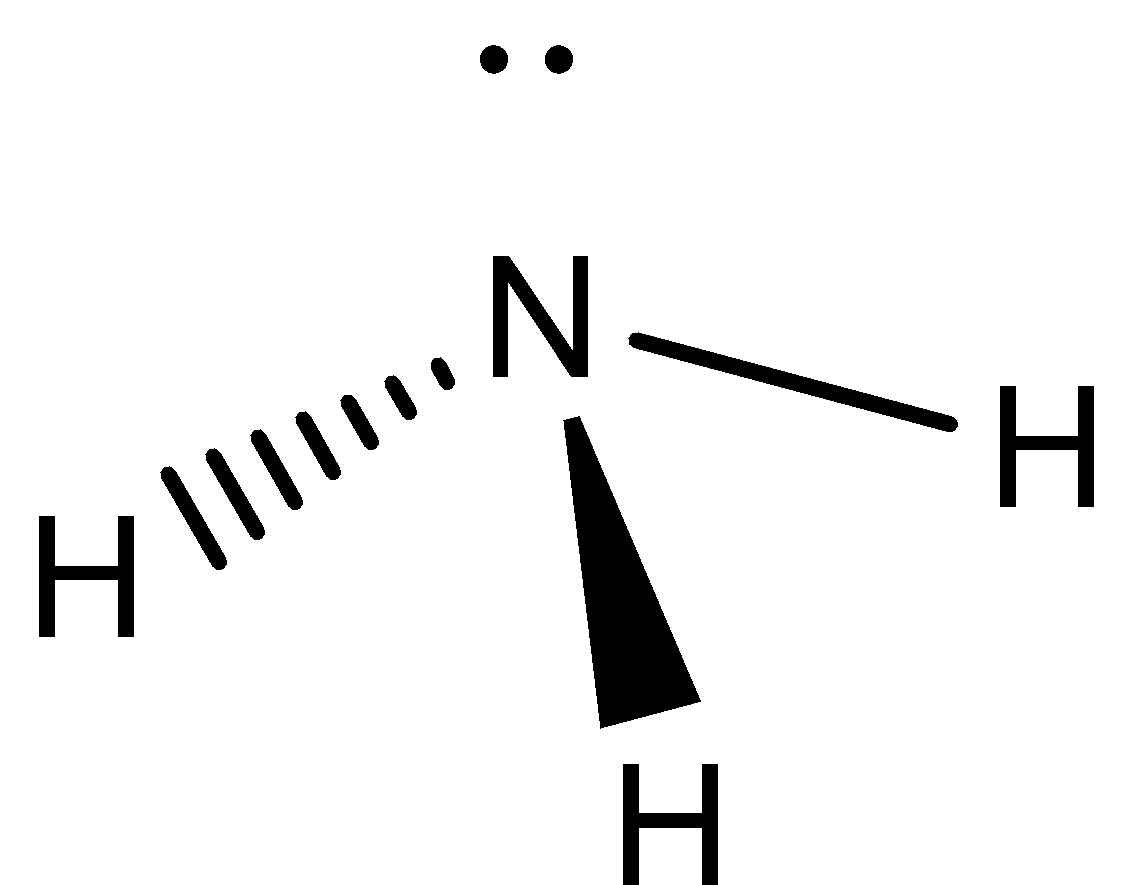
Starting with the Lewis dot structure we know that nitrogen has five valence electrons whereas hydrogen has one valence electron. Thus the total valence electrons will be eight since three hydrogen atoms are present. So, nitrogen is a central atom having three atoms bonded with three hydrogen atoms and left with two valence electrons in total. These two valence electrons will behave as a lone pair of electrons. During hybridization, one s and 3 p orbital form four hybrid orbitals that participate to give the hybridization as sp3. There are two elements in NH3 hydrogen and nitrogen. Hydrogen is a group IA element and has only one electron in its last shell (valence shell).
Note:
There are two elements in NH3; hydrogen and nitrogen. Hydrogen is a group IA element and has only one electron in its last shell (valence shell). Nitrogen is a group VA element in the periodic table and contains five electrons in its last shell. Nitrogen is a group VA element in the periodic table and contains five electrons in its last shell.
