Question
Question: What is the Lewis structure for the resonance form of \[Cl{O_2}^ - \]?...
What is the Lewis structure for the resonance form of ClO2−?
Solution
Lewis structures are the diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that can exist in the molecule. It is found that these are called electron dot structures. It basically adds up two lines between the atoms that show shared pairs.
Complete answer:
Chlorine dioxide has an odd number of valence electrons and is considered a paramagnetic radical. The compound is composed of a central chlorine atom because chlorine is less electronegative than oxygen. The two oxygen atoms connected via covalent bonds.
Chlorine dioxide has two resonance structures with a double bond and three electrons on the other. Both resonance structures have a bent molecular geometry with an O=Cl−O bond angle of 117.6∘.
Every atom has a formal charge; we can reduce the number of formal charges by moving a lone pair of electrons from oxygen to form a Cl=O double bond. This gives us two new structures in which one oxygen atom has a formal charge −1 and the other atom has a formal charge of 0.
The two resonance structure of ClO2− are:
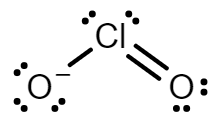
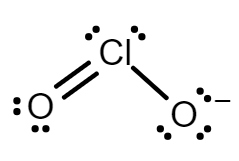
Note:
Lewis structure mainly describes the structure of molecules with the help of symbols for the chemical species and also dot symbols. It is found that octet rule exists because the atoms of most of the elements become more stable by attaining the electronic configuration of an element.
