Question
Question: What is the lewis structure for \( OC{N^ - } \) ?...
What is the lewis structure for OCN− ?
Solution
Hint : Lewis structure is also known as lewis dot formulas, it is the diagram that represents valence electrons of atoms within the molecular structure. These structures help to visualise the valence electrons of atoms and to know if they exist as lone pairs or not.
Complete Step By Step Answer:
O−C−N
We will add two double bonds and one triple bond between atoms
O=C=N↔O≡C−N↔O−C≡N
Now we will show unshared electron pairs around every atom so that an octet of electrons will be shown around it.
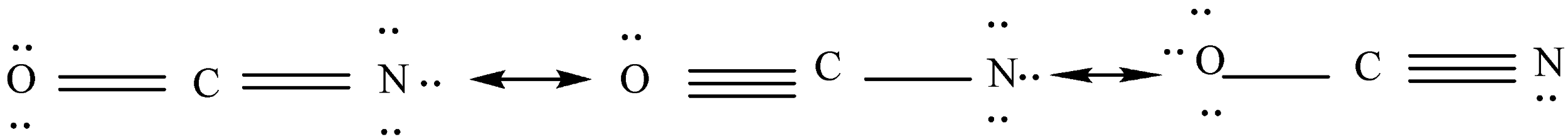
We will calculate the formal charge of each atom according to the last step.
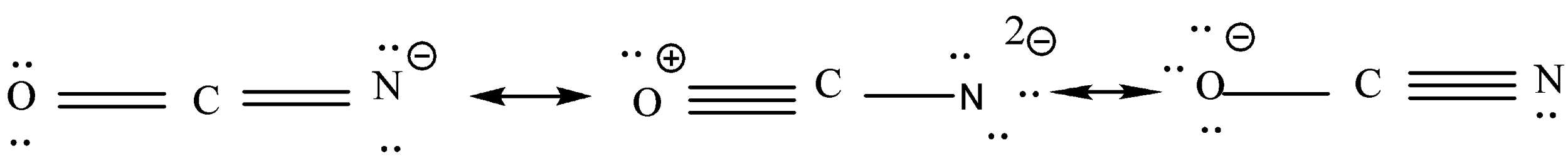
This is resonance structure.
Note :
OCN− has a triple bond between carbon and nitrogen atoms and that is why it can be termed as an ambidentate ligand. This cyanate ion is stable. Its electron pair geometry is tetrahedral and the molecular geometry is trigonal pyramidal.
