Question
Question: What is the Lewis Dot Diagram for Platinum?...
What is the Lewis Dot Diagram for Platinum?
Solution
Hint : Lewis dots are the structure which helps one to identify the number of electrons present in the Valence shell of any element. These Lewis symbols and Lewis structures help visualize the valence electrons of atoms and molecules, whether they exist as lone pairs or within bonds.
Complete Step By Step Answer:
To Assign dot structure, we will consider only the valence shell electrons of elements.
$$$$
1.The first step is to write the chemical symbol. Here it isPt.
The atomic number of platinum(Pt)is78. In the periodic table, this falls in the10th and 6thperiod.
Platinum has electronic configuration of: 78Pt=1s22s22p63s23p64s23d104p65s24d105p66s24f145d8
Or the abbreviated configuration is: Pt=[54Xe]6s24f145d8
It is clear that platinum has only 8 electrons in the last shell which is the valence shell.
2.Second step is to find the valence electron, that is8.
3. Third is the last step, is to draw the structure.
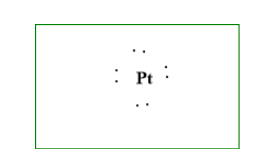
Additional Information:
The columns, or groups, table are in the periodic table used to determine the number of valence electrons for each element. Each column of the table contains elements that have the same number of columns from the left edge of the table tells us the exact number of valence electrons for that element.
Note :
The noble gas is chemically stable and has a full valence level of electrons. Other elements react in order to have the full valence level of electrons. Lewis symbols represent the valence electrons as dots surrounding the elements symbol for the atom. Once you can draw a Lewis symbol for an atom, you can use this knowledge to create dot structure of molecules.
