Question
Question: What is the lattice energy of Calcium Chloride?...
What is the lattice energy of Calcium Chloride?
Solution
Hint : The Born-Haber cycle is a very useful method in calculating the lattice enthalpy of any compound. To find out the lattice enthalpy we need the total ionization energy when the compound is prepared from its constituent atoms or ions. We also need sublimation energy, dissociation energy, ionization energy and electron affinity values.
Complete Step By Step Answer:
Let us first consider the Born-Haber Cycle of CaCl2 . Only by seeing this we can calculate the lattice energy of the compound.
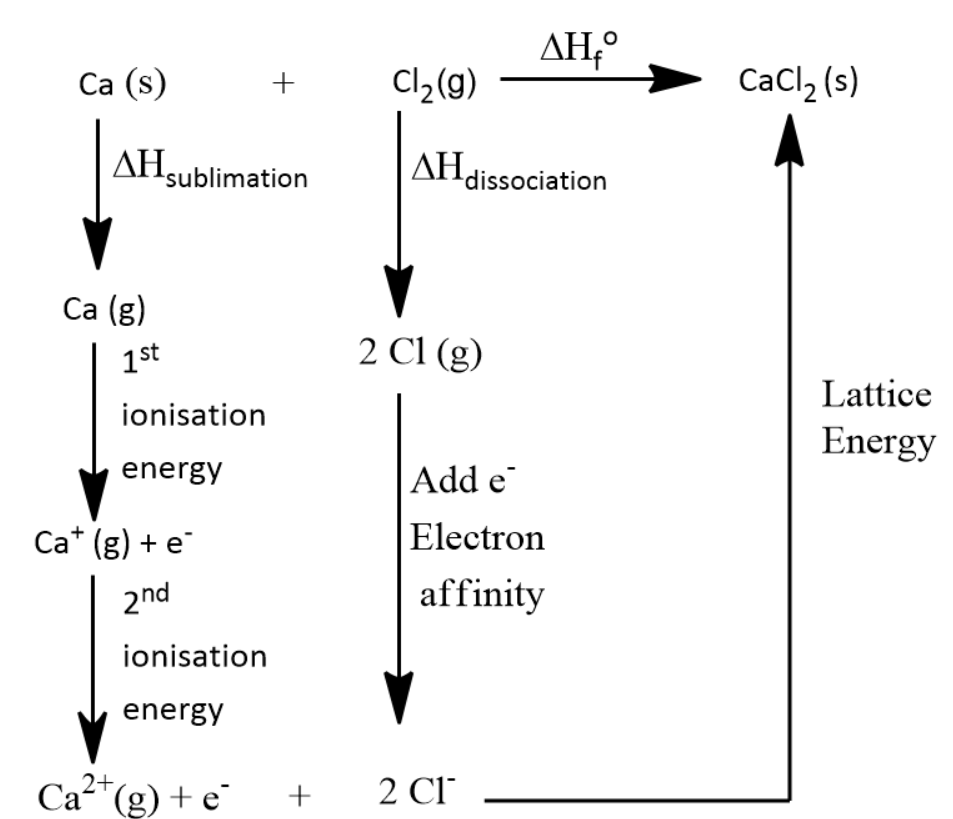
By the above diagram we can say that
Standard enthalpy of formation ( ΔHfo ) = lattice energy + (2 × Electron affinity for chlorine) + Bond Energy of Chlorine gas + first and second ionisation energy of calcium + Sublimation energy of Calcium solid.
ΔHsublimation = 121 kJ
1st Ionization Energy of Ca = 589.5 kJ
2nd Ionization Energy of Ca = 1145 kJ
Bond Energy of Cl2 = 242.7 kJ
Electron Affinity for chlorine = 2 × (−349 kJ )
Standard Enthalpy of Formation of CaCl2 (s) = −795 kJ
We can substitute these values in the equation to find out the lattice energy
⇒−795 kJ = Lattice Energy + 2×(−349 kJ) + 242.4 kJ + 1145 kJ + 589.5 kJ + 121 kJ
⇒Lattice Energy = −795 kJ−2×(−349 kJ)−242.4 kJ−1145 kJ−589.5 kJ−121 kJ
⇒Lattice Energy=−2195.2 kJ/mol
This is how we find the lattice energy of calcium chloride by using Born-Haber cycle.
Note :
We can also use another method to find out the lattice energy of a compound
This method is by using the Born-Lande Equation, given by
Lattice Energy=4πεorNA×M×z+×z−×e2(1−n1)
Where NA is the Avogadro number
M is the Madelung Constant
z+,z− are the charges of cation and anion respectively
e is the electronic charge
εo is permittivity of free space
r is the radial distance
n is the born exponent.
