Question
Question: What is the IUPAC name of Iso-octane? A) Octane B) \[2,2,4 - Trimethylpentane\] C) \[2,5 - Dim...
What is the IUPAC name of Iso-octane?
A) Octane
B) 2,2,4−Trimethylpentane
C) 2,5−Dimethylhexane
D) 2−methylheptane
Solution
We have to remember that the isoforms are the simplest form of isomers having all carbons in a straight chain or branched if a chain is long, except for one carbon which restricts the continuation of carbon atom in a straight or branched chain. For example:
Iso-butane, Iso-pentane.
Complete step by step answer:
We have to remember that an octane is a hydrocarbon having a chemical formula C8H18. It comes under the class of alkane as it has all single C-H bonds.
There are many isomers of octane which differ in the structural formula or you can say the position of Carbon atoms.
Option A) this is an incorrect option as octane is not an iso structure of octane.
Octane chemical formula is C8H18
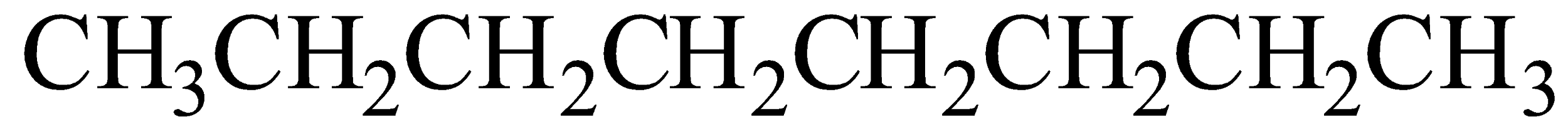
Option B) This is a correct option as 2,2,4−Trimethylpentane is IUPAC name of iso-octane.
Structure of iso octane is as follows:
(CH3)3CCH2(CH3)2 or
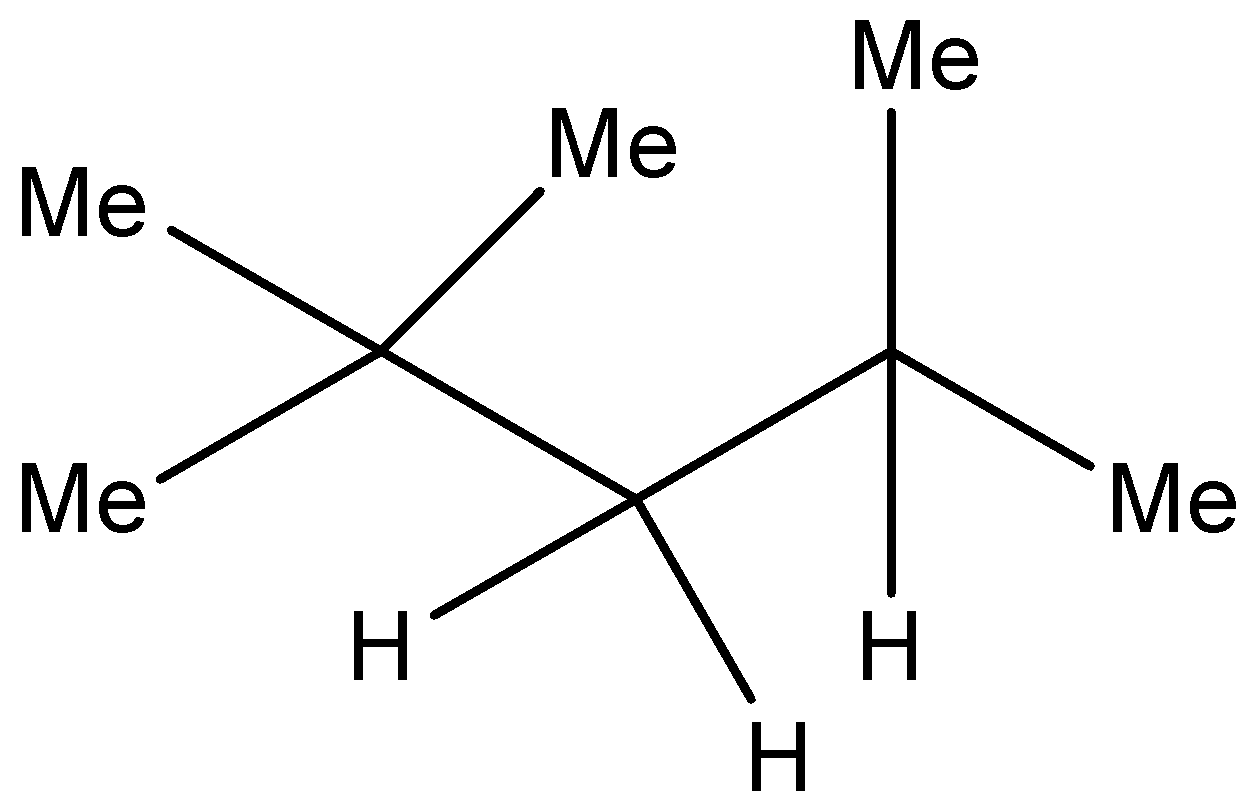
This is the most common isomer of octane. This chemical is used to obtain the octane rating of any fuel.
Octane is less dense than water and therefore is insoluble in it. Also octane is a component of gasoline.
Option C) this is an incorrect option as 2,5−Dimethylhexaneis an isomer of octane but it does not belong to iso structure.
The structural formula for 2,5−Dimethylhexane is as follows:
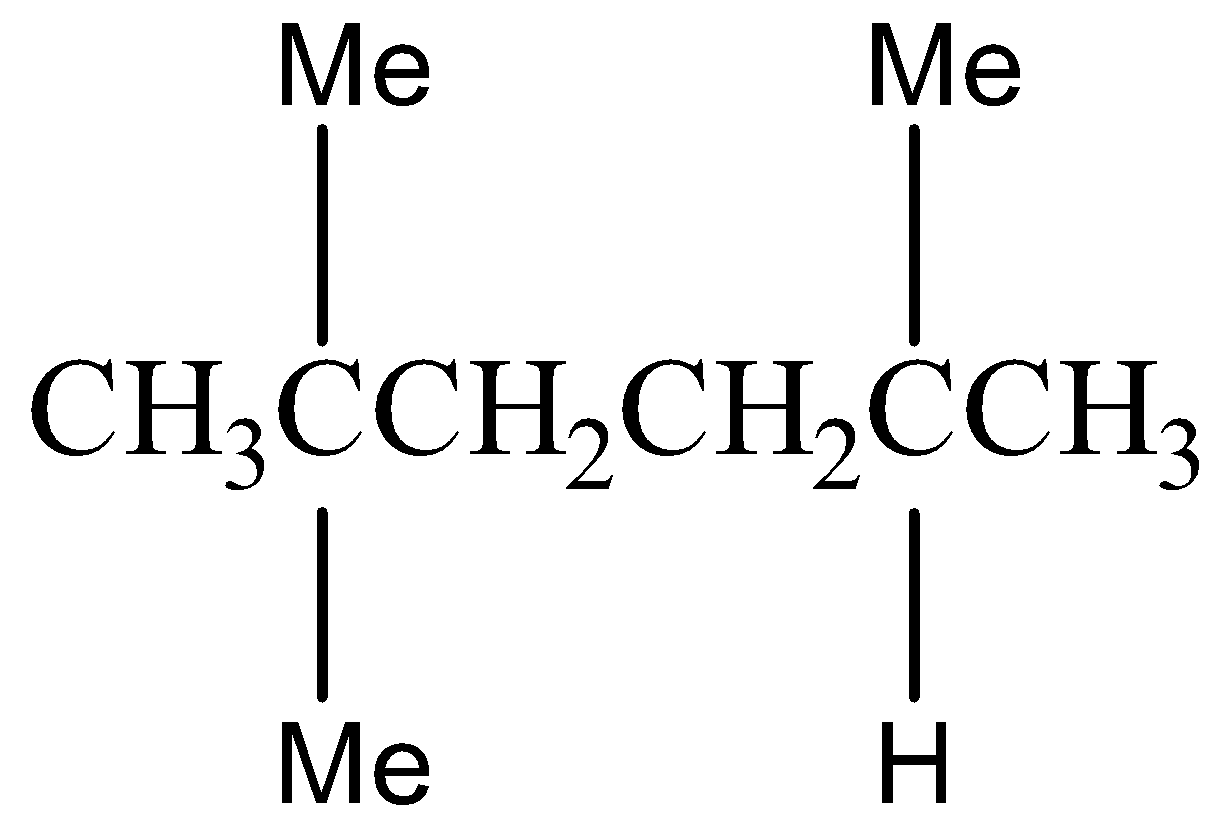
Option D) this is an incorrect option as 2−methylheptane is an isomer of octane but it does not belong to iso isomer.
Structural formula of 2−methylheptane is as follows:
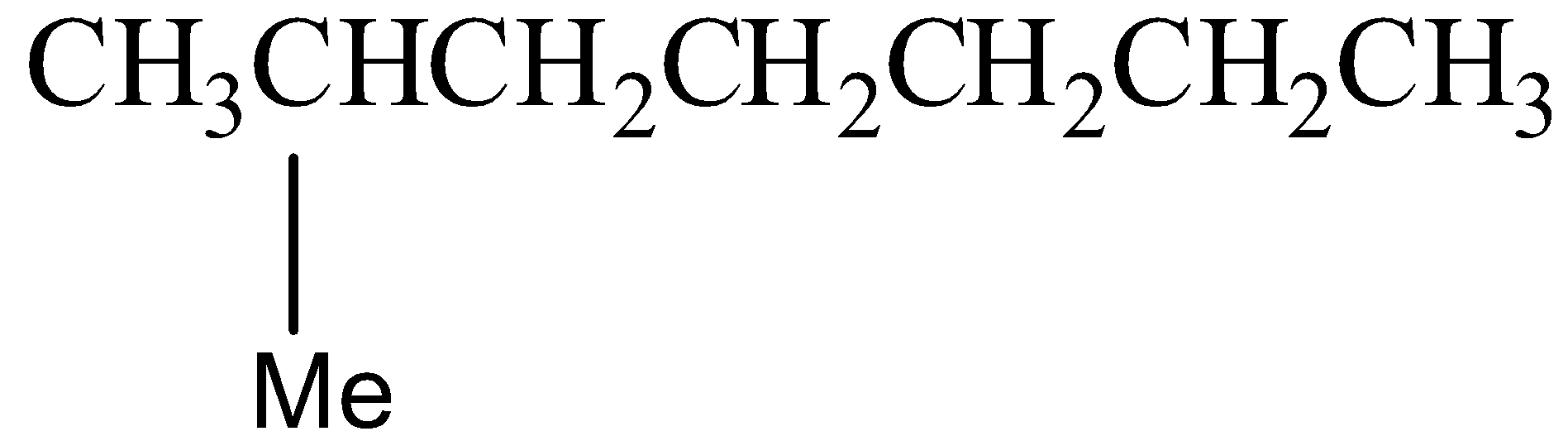
Note: We have to remember that the octane has several isomers that are structural isomers but the most common in it is iso-octane having a chemical name as 2,2,4−Trimethylpentane.
