Question
Question: What is the IUPAC name of \(C{{l}_{2}}CHC{{H}_{2}}OH\)? (A) 2,2-Dichloro ethanol (B) 2,2-Dichlor...
What is the IUPAC name of Cl2CHCH2OH?
(A) 2,2-Dichloro ethanol
(B) 2,2-Dichloro ethanol
(C) 2,2-Dual Chlorine ethanol
(D) 2,2-Chloro Chloro ethanol
Solution
Draw the expanded structure of the given compound to understand the bonding of halogen to the carbon atom. Try recalling the IUPAC conventions followed while naming a compound. The numbering of carbon atoms starts from the carbon atom having a functional group.
Complete step by step answer:
We will now write the expanded structure of the compound.
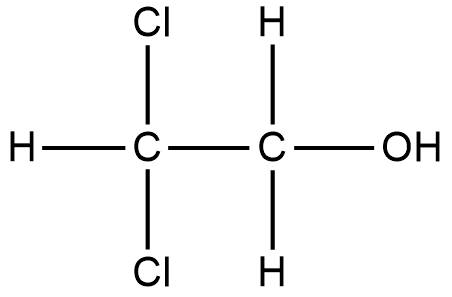
The above consists of 2 carbon atoms. The hydroxyl (OH) group gets more priority than the halogen functional group. So, the hydroxyl group becomes the main functional group and the halogen atoms are substituent groups.
The carbon having the hydroxyl group gets the number 1 and the carbon having halogen atoms gets number 2.
Since 2 chlorine atoms are attached to the second carbon, the prefix becomes 2,2-dichloro.
We will now write the complete name for the organic compound:
IUPAC name - 2,2-Dichloro ethanol.
So, the correct answer is “Option A”.
The International Union of Pure and Applied Chemistry is an international federation of National Adhering Organizations that represents chemists in the individual countries. IUPAC is registered in Zürich; Switzerland and its administrative office is called IUPAC secretariat.
Note: In the above question it was easy to choose the longest carbon chain and the main functional group. However, when we have to choose between the main functional group and longest chain, we give priority to the functional group. The above explanation is shown below:
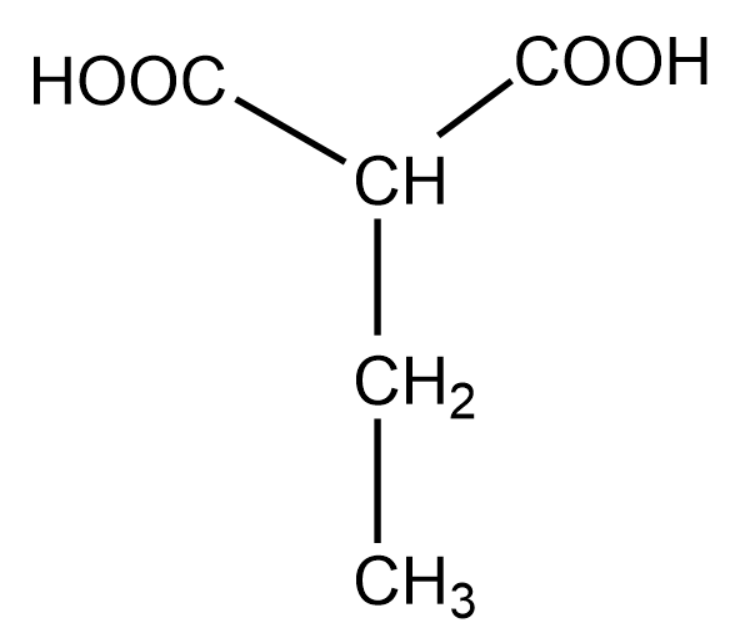
For the above organic compound, the IUPAC name is 2-ethylethan 1,3-dioic acid.
