Question
Question: What is the IUPAC name of \(C{{H}_{3}}-C(C{{H}_{3}})(OH)-C(O)-C{{H}_{3}}\)? (a)- Hetane-1, 3, 5-tr...
What is the IUPAC name of CH3−C(CH3)(OH)−C(O)−CH3?
(a)- Hetane-1, 3, 5-tricarboxylic acid
(b)- Pentane-1, 3, 5-tricarboxylic acid
(c)- 3-Hydroxy-3-methyl butan-2-one
(d)- 3-Butyl-1, 4-hexadien-6-al
Solution
When the compound is given in the condensed form, expand it because it will be easier for naming the compound. The numbering of the compound will start from the carbon atom attached ketone group. When the naming is done, the preference will be given to the ketone group and then the hydroxyl group.
Complete step-by-step answer: The compound given for the naming is CH3−C(CH3)(OH)−C(O)−CH3, this compound is in condensed form. So, let us expand this formula for convenience. The expanded form is:
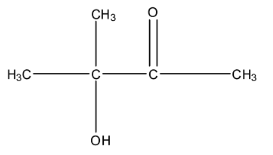
So, we can see that the longest chain of the carbon atom contains four atoms. Therefore, this chain is butane. Now, we have to do the number in this chain. There are two functional groups in this chain, i.e., the ketone group and hydroxyl or alcohol group. The numbering of the carbon chain will start from the ketone side because the ketone group is given preference over the hydroxyl group.
The numbering is shown below:
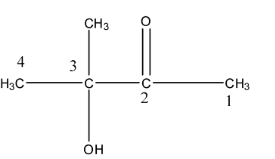
At the second position, the ketone functional group is present, at the third position, the hydroxyl or alcohol group is present, and at the third position, methyl substituent is present. So, while naming the ketone will be named in the suffix part and the alcohol or the hydroxyl group will be named in the prefix part because the preference is given to ketone over the hydroxyl group. Therefore, the name of the compound will be 3-Hydroxy-3-methyl butan-2-one.
Hence, the correct answer is an option (c).
Note: While naming the compound the alphabetic order should be considered not the numbering. In 3-Hydroxy-3-methyl butan-2-one, the hydroxyl is named before the methyl group because h comes before m.
