Question
Question: What is the IUPAC name of \({{C}_{10}}{{H}_{12}}O\)?...
What is the IUPAC name of C10H12O?
Solution
The given question can be solved only when we are able to draw the structure of the given molecular formula in such a way that no valency is left unsatisfied. Thus, in order to do so, we first need to find out the degree of unsaturation in the molecule, and then accordingly make the structure of the molecule and then give it the IUPAC naming.
Complete answer: First of all, we need to calculate the degree of unsaturation present in the molecule. Degree of unsaturation refers to any ring or double or triple bond present in the molecule.
Degree of unsaturation or double bond equivalent (DBE)=C+1−2H+X−N ;
Here, C= number of carbon atoms= 10
H= number of hydrogen atoms= 12
X= number of halogen atoms= 0
N= number of nitrogen atoms= 0
Thus, double bond equivalent = 10+1−212=11−6=5
Since the DBE is greater than 4, there is definitely a phenyl ring present.
Also, since the phenyl ring has the DBE=4 , there is 1 more unsaturation. This can be inferred to as either a double bond or a carbonyl group. Since no information is further given about the molecule, we consider it to have a carbonyl group.
Hence, the structure so formed of the molecule is:
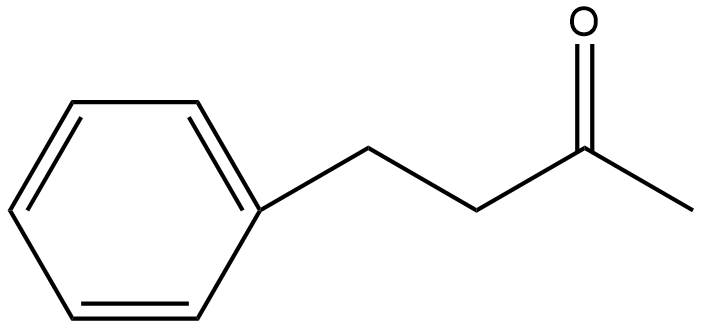
Therefore, the IUPAC name of the given molecule is 1-phenylbutan-3-one.
Note:
The following molecular formula given can have other isomers too. And it need not be carbonyl only, it can also take form in the functional group of hydroxyl group too. Also, it can exhibit position isomer as well chain isomer. However, since no such restrictions or options are given in the question, the question can be approached with any acceptable approach.
