Question
Question: What is the IUPAC name for the following compound \[?\] 
A.5−chloro−3−methylhexanone
B.1−chloro−1,3−methyl−4−pentanone
C.5−chloro−3,5−methyl−2−pentanone
D.5−chloro−3−methyl−2−hexanone
Solution
First we know the full form of IUPAC is “International Union of Pure and Applied Chemistry”. It is an international organization which mainly refers to the systematic approach taken for the nomenclature of organic compounds as per the recommendations. The IUPAC name of any organic compound essentially consists of three parts, which are stem name, prefix and suffix.
Complete answer:
According to the IUPAC, the nomenclature of (alkanes)compounds must follow these steps:
Step 1: The longest chain can be identified and subsequently named. The longest chain of carbon atoms, to be in the form of a straight chain or a chain of any other shape.
Step 2: The carbon atoms belonging to the long chain must be numbered using natural numbers and beginning from the end in which the lowest number is assigned to the carbon atom which carries the substituents.
Step 4: Identify the prefixes of same substituent. If two or more similar substituents (alkyl groups) are present in the compound, then the words 'di', 'tri' 'tetra' and so on are used to specify the number of times those appear in the long chain.
Step 5: If the organic compounds contain multiple substituents, then the different substituents are arranged in alphabetical order of base names. The complex substituents of compounds having branched structures must be named as substituted alkyl groups whereas the carbon which is attached to the substituent group is numbered as one. These branched and complex substituents must be written in brackets.
Hence the general format of the IUPAC name of the compound can be written as: Locant+Prefix+Root+Locant+Suffix
Where root indicates the total number of carbon atoms present in the longest carbon chain belonging to the compound. For example, ‘pent’ refers to a chain with 5 carbon atoms. Prefixes are added before the root and it indicates the presence of side chains in the given compound. The prefixes can be broadly classified into primary prefixes and secondary prefixes.
In the given compound, the long chain contains 6 carbon atoms. Numbering the carbon atoms belong to long chain of the given compound shown as follows
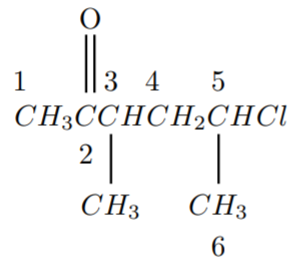
Hence the substituent ketone at C-2, methyl group at C-3, and chloro bond at C-5. Since a long chain contains 6 carbons, we add the prefix as “hex”.
Hence the correct option is (D) 5−chloro−3−methyl−2−hexanone.
Note:
The suffix in IUPAC nomenclature is usually a functional group belonging to the molecule which follows the root of the name. It can be further divided into a primary and a secondary suffix.
A Primary Suffix is written immediately after the word root. For example, in the case of alkanes the suffix is ‘ane’. A Secondary Suffix written after the primary suffix is written. For example, compounds with an alkane and alcohol group attached to them will be named as an alkanol, with ‘ol’.
