Question
Question: What is the IUPAC name for \[{H_3}P{O_3}\]?...
What is the IUPAC name for H3PO3?
Solution
IUPAC stands for International Union of Pure and Applied Chemistry. It is the world authority on the chemical nomenclature as well as terminology. In this question we have to tell the IUPAC name ofH3PO3 which is an oxyacid of phosphorus. It is a diprotic acid which readily ionizes two protons.
Complete step by step answer:
IUPAC provides consistency to the names of organic compounds. It enables every compound to possess a unique name, which otherwise is not plausible with the common names. The chemical structure for H3PO3is shown below:
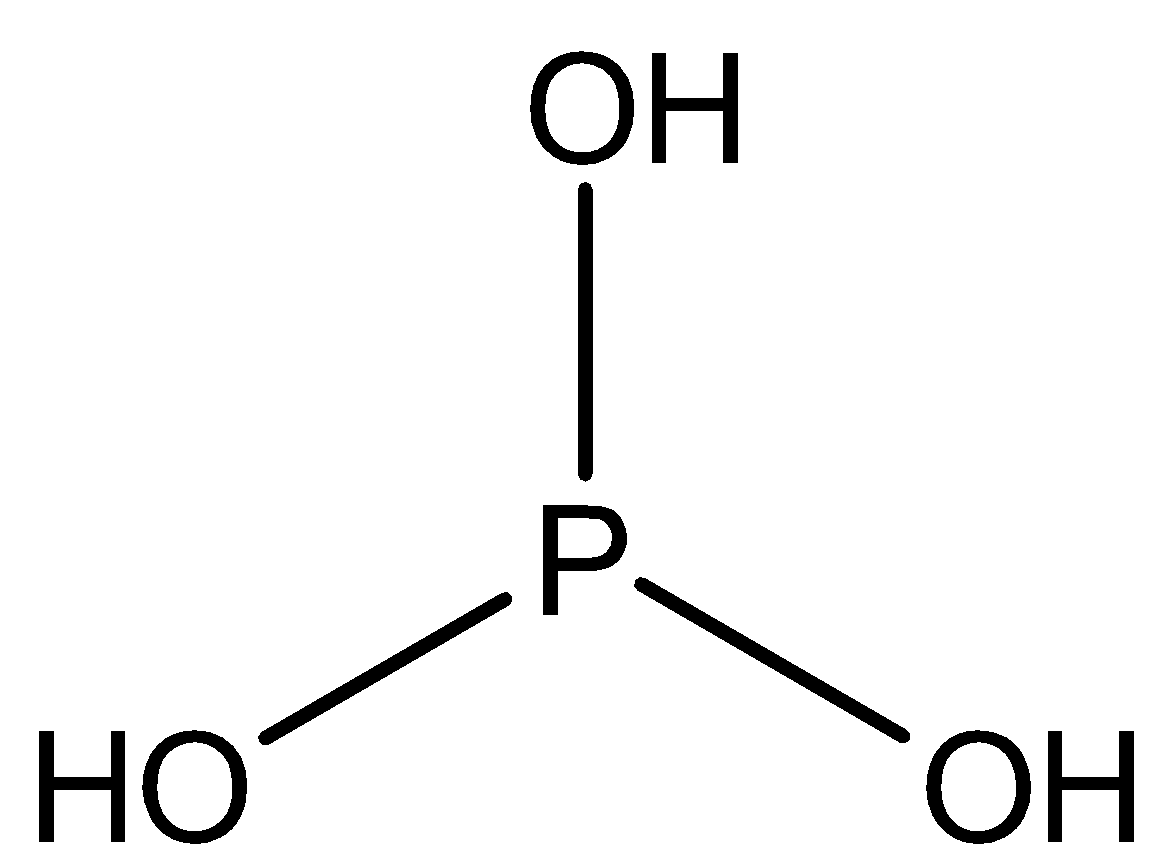
H3PO3can be more clearly explained with its structural formulaHPO(OH)2. In solid state, HP(O)(OH)2is actually tetrahedral withP−H bond of1.32pm, one short P=O bond of148pmwhile two long P−O(H)bonds of154pm. It occurs in equilibrium with a minor tautomer i.e. P(OH)3. According to IUPAC nomenclature,H3PO3is called phosphorous acid, while the dihydroxy form is known as phosphonic acid. The reduced phosphorus compounds are only named with an ending "ous".
Hence, the IUPAC name forH3PO3is phosphorous acid.
Additional information:
Phosphorous acid (H3PO3), also known as orthophosphorous acid, is generally employed as a reducing agent in the chemical analysis. It is actually either a colourless or a yellowish crystalline substance having a melting point of around73∘C, or163∘F and a taste similar to garlic. It is considered as an unstable compound which readily absorbs moisture, and gets converted to the phosphoric acid (H3PO4) in the presence of oxygen usually when heated above 180 ∘C (i.e.360 ∘F). Phosphorous acid (H3PO3) generally leads to the formation of salts called as phosphites, which are employed as reducing agents.
Note: H3PO3 is generally prepared by dissolving either tetraphosphorus hexoxide (P4O6) or phosphorus trichloride (PCl3) in water. The reactions are demonstrated below:
P4O6+6H2O→4H3PO3 PCl3+3H2O→3HCl+H3PO3
