Question
Question: What is the isotope symbol for Iodine-131?...
What is the isotope symbol for Iodine-131?
Solution
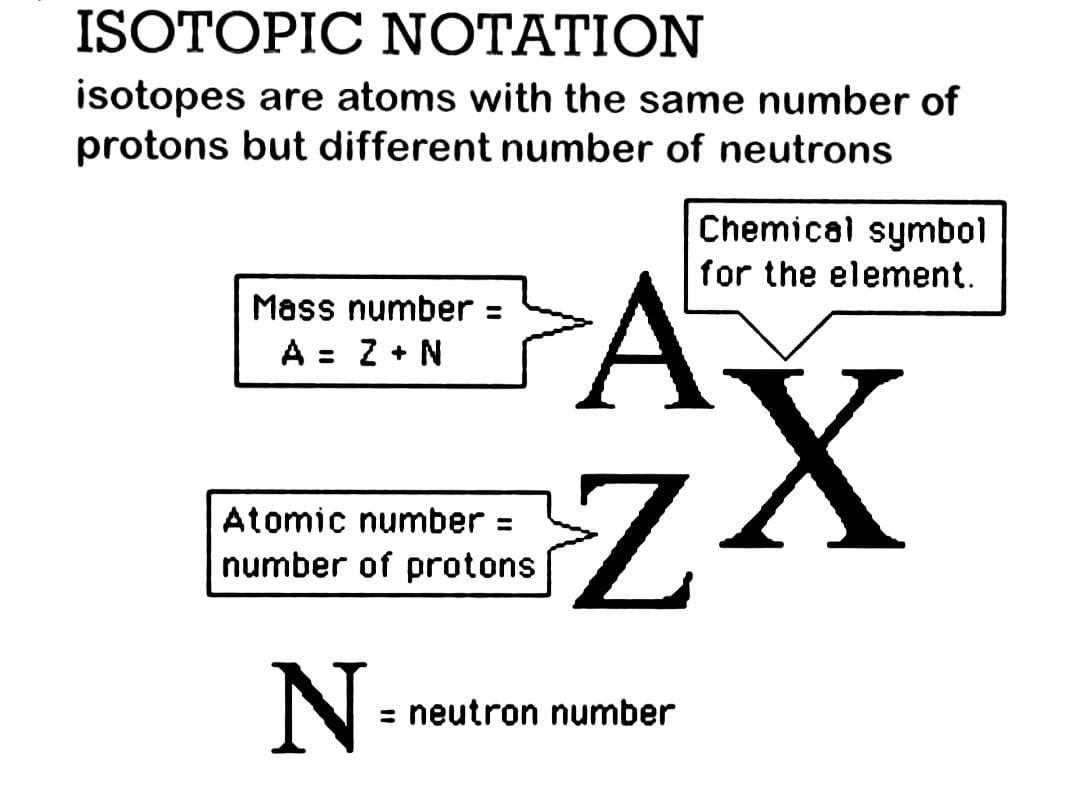
Hint : In order to answer this question, to write the symbol for an isotope, put the atomic number as a subscript to the left of the atomic symbol and the mass number (protons plus neutrons) as a superscript.
Complete Step By Step Answer:
Iodine-131 has the isotope symbol of 53131I
Explanation:
The isotope sign, also known as isotope notation or nuclear notation, represents an isotope's atomic number ,Z, as well as its mass number, A . On the left-hand side of the element's chemical symbol, X, the mass number is expressed as a superscript. On the left-hand side of the element's chemical symbol, the atomic number (number of protons) is expressed as a subscript.
The mass number (A) of Iodine-131 is 131 , and the atomic number (Z) is 53 . As a result, the nuclear symbol for Iodine-131 is 53131I .
Additional Information:
Iodine-131 is a significant iodine radioisotope. It has an eight-day half-life in terms of radioactive decay. Nuclear energy, medical diagnostic and treatment operations, and natural gas production are all linked to it. It also plays an important function as a radioactive isotope found in nuclear fission products, and it contributed significantly to the health risks associated with open-air atomic bomb testing.
Note :
Iodine-131 is a gamma-emitting radioactive industrial tracer that is widely used. To determine the injection profile and location of fractures caused by hydraulic fracturing, radioactive tracer isotopes are injected with hydraulic fracturing fluid.
