Question
Question: What is the Isothermal process?...
What is the Isothermal process?
Solution
Any process in which temperature remains the constant is called an Isothermal process or we can say the process in which ΔT=0 is called an Isothermal process.
Where ΔT is the change in temperature.
Complete step by step answer:
Isothermal Process: An isothermal process may be defined as a thermodynamic process in which the temperature remains constant.
In an isothermal process ΔT=0 always, where ΔT is the change in temperature.
The P-V diagram of an isothermal process is shown below -
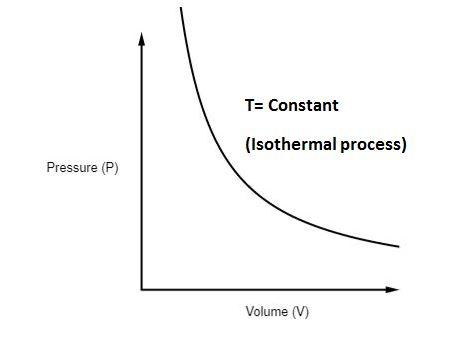
Some examples of isothermal process are as follows:
All the processes which are taking place inside a refrigerator are isothermal processes as there is no change in its internal temperature.
The melting of ice at zero degrees Celsius is also an isothermal process.
An ideal Carnot engine also works on an isothermal process.
The amount of work done during an isothermal process is given by :
W=nRTlnV1V2=2.303nRTlogV1V2
Since P1V1=P2V2 (Boyle’s law)
Therefore work done can also be written as,
W=nRTlnP2P1=2.303nRTlogP2P1
Where n is the number of moles of a gas, R is the universal gas constant, T is the temperature, P1 and P2 are the initial and final pressures respectively & V1 and V2 are the initial and final volumes respectively.
Note:
Keep in mind that in an isothermal process only temperature remains constant i.e. ΔT=0. If the gas is an ideal gas then change Internal energy also becomes zero in the isothermal process i.e. ΔU=0 but the change in heat will never equal to zero i.e. ΔQ=0. Moreover, the process in which heat remains the constant i.e. ΔQ=0 is called as an adiabatic process.
