Question
Question: What is the ionic formula of sodium carbonate?...
What is the ionic formula of sodium carbonate?
Solution
Hint : When sodium atom is converted to its ion it gets a charge of +1 and similarly carbonates ion has a charge of −2. Here sodium acts as a cation and carbonate acts as an anion. To construct the ionic formula, we will use the valency of each ion and then balance it.
Complete Step By Step Answer:
In sodium carbonate, sodium ion (Na+) has a charge of +1 and carbonate ion (CO32−) has a charge of −2. We know that the valency of an ion is same as the charge on that ion. Hence the valency of Na+ is 1 and the valency of CO32− is 2.
Now, we will use the crossover rule and cross the valency of each element to the other element as follows:
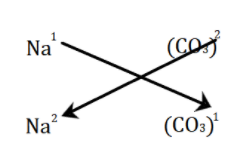
Hence, the valency is swapped and is balanced accordingly. Therefore, the ionic formula of sodium carbonate is Na2(CO3).
Additional information: Sodium carbonates is a diazonium salt of carbonic acid and is also known as washing soda, soda ash or soda crystals. It is white powder in its pure form and is odorless. It has three hydrate forms, namely,
Sodium carbonate decahydrate (Na2(CO3).10H2O)
Sodium carbonate heptahydrate (Na2(CO3).7H2O)
Sodium carbonate monohydrate (Na2(CO3).H2O)
Simple sodium carbonate is an anhydrous salt and is manufactured during the last step of the Solvay process. Anhydrous sodium carbonate is also known as calcined soda.
Note :
The ionic formula of a compound tells us about the mole-to-mole ratio of different elements present in that compound. Valency of an atom or ion can be understood as the number of electrons gained, lost or shared during a chemical reaction to form bonds with other atoms or to attain stability.
