Question
Question: What is the hybridization state of each carbon atom in \({H_2}C = C = C{H_2}\)?...
What is the hybridization state of each carbon atom in H2C=C=CH2?
Solution
We know that the redistribution of energy of orbitals of individual atoms takes place to form orbitals of equivalent energy that happens when the two atomic orbitals combine to form hybrid orbital in a molecule and this process is known as hybridization.
Complete answer:
In organic chemistry, the hybridization state of the carbon atom is determined by the number of sigma bonds and pi bonds associated with that carbon atom. If a carbon atom consists of Only sigma bonds which means hybrid orbitals show head-to-head overlapping and match the symmetry of atom’s atomic orbital. So, the carbon atom with only sigma bonds is sp3 overlapping. If a carbon atom consists of one pi and three sigma bonds, then the hybridization of carbon atom is observed to be sp2 and if the carbon atom consists of two sigma and two pi bonds, then the carbon atom is said to be sp hybridized.
Now, for determining the hybridization of each carbon atom in the given compound, we first need to count the number of sigma and pi bonds associated with each carbon atom. The sigma and pi bonds in the given organic compound are represented as follows:
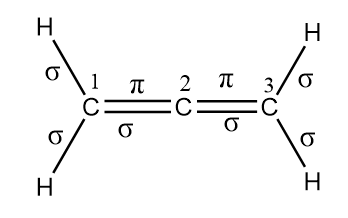
Therefore, the number of sigma and pi bonds for each carbon atoms are as follows:
Carbon-1: Sigma bonds =3 and pi bonds =1
Carbon-2: Sigma bonds =2 and pi bonds =2
Carbon-3: Sigma bonds =3 and pi bonds =1
Thus, we can conclude that the hybridization state of each carbon atom in the given compound is as follows:
Carbon-1: sp2
Carbon-2: sp
Carbon-3: sp2
Note:
Remember to never make assumptions of the hybridization state of carbon just by looking at the type of bond i.e., single, double or triple bond as it may lead to inaccurate results. As per above compound, the central carbon is bonded to two double bonds but it is not sp2 hybridized instead it is sp hybridized. Thus, always calculate the number of sigma and pi bonds before stating the hybridization state.
