Question
Question: What is the hybridization state of B and N in inorganic benzene respectively? (A) \[s{p^2} and {\r...
What is the hybridization state of B and N in inorganic benzene respectively?
(A) sp2andsp3
(B) sp3andsp2
(C) Bothsp2
(D) Bothsp3
Solution
Borazine is called as the inorganic benzene, this is because borazine resembles benzene in the structure and few properties. If we know the hybridisation of the carbon atom in benzene, it would be easy for us to compare.
Complete answer:
Before we directly move into the hybridisation state of B and N in inorganic benzene, we must know what a borazine is. Borazine is a chemical compound with the molecular formula of B3N3H3. Borazine is a colourless liquid. It has an aromatic smell. It was first prepared from the chemical reaction of diborane and ammonia in the year 1926. It was called inorganic benzene because it is isoelectronic with benzene. Borazine is having a structure which is similar to the benzene, i.e. planar hexagonal structure. In borazine structure, Boron and Nitrogen atoms are arranged alternatively.
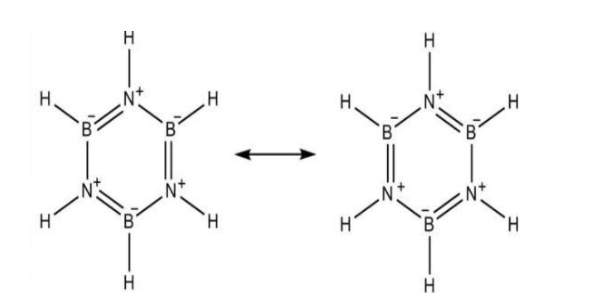
In the borazine structure, Boron is bonded to three atoms one hydrogen and two Nitrogen atoms. As it is bonded to three atoms, each boron atom in borazine will be having sp2 hybridisation and trigonal planar structure.
The nitrogen atom in borazine is bonded to three atoms namely two boron atom and one hydrogen atom. Like boron, as nitrogen is also bonded to three atoms, the hybridization will also be sp2.
Therefore, both B and N in inorganic benzene i.e. borazine will be having sp2 hybridisation.
Hence the correct answer will be option (C) Bothsp2.
Additional information:
Difference between benzene and borazine
| BORAZINE | BENZENE |
|---|---|
| Molecular formula- B3N3H3 | Molecular formula - C6H6 |
| Inorganic compound | Organic compound |
| Contains B and N | Contains only C |
| Reactive | Less reactive |
| Not a perfect hexagon | perfect hexagon |
Note: As we know that benzene and borazine are having similar structure, i.e. hexagonal structure. In benzene, the carbon atom is having sp2 hybridisation. Therefore, like benzene, in borazine, N and B should also have sp2 hybridisation.
