Question
Question: What is the hybridization of phosphorus trichloride? (a) \(s{{p}^{3}}\) (b) \(s{{p}^{2}}\) (...
What is the hybridization of phosphorus trichloride?
(a) sp3
(b) sp2
(c) sp
(d) None of these
Solution
Phosphorus has five electrons present in its valence shell and each chlorine atom has one unpaired electron present in its valence shell. Three phosphorus atoms form a bond with the fluorine atom and rest is present as a lone pair. Now you can easily identify the hybridization of phosphorus trichloride.
Complete answer: First of let’s discuss what is hybridization. By the term hybridization we mean the phenomenon of intermixing of the orbitals of slightly different energies so as to redistribute their energies to give a new set of orbitals of equivalent energies and shape.
The conditions for the hybridization are as follows:
(i) only the orbitals present in the valence shell of the atom are hybridized.
(ii) the orbitals undergoing hybridization should have only a small energy difference. The orbitals which greatly differ in energies cannot take part in hybridization.
(iii) It is not necessary that only half-filled orbitals participate in hybridization . In certain cases, even half-filled orbitals of the valence shell participate in hybridization.
Now considering the statement as;
Phosphorus has atomic number as 15 and has five electrons in its outermost valence shell which are unpaired.
In phosphorus pentachloride, phosphorus has five electrons in its valence shell and forms three sigma bonds with the three fluorine atoms and two electrons are present as lone pairs on the phosphorus atom. Thus, the hybridization of the molecule is sp3( three sigma bonds and one lone pair).
The shape of phosphorus trichloride is:
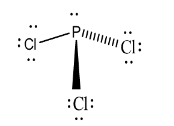
Hence, option (a) is correct.
Note: Always remember that the number of hybridized orbitals formed is always equal to the number of orbitals that gets hybridized. The hybridized orbitals are always equivalent in energy and shape and are more effective in forming stable bonds than the pure atomic orbitals .
