Question
Question: What is the Hybridization of \(C{H_4},N{H_3},{O_2},{N_2}\& {H_2}O\)?...
What is the Hybridization of CH4,NH3,O2,N2&H2O?
Explanation
Solution
Hybridisation is the process of combining atomic orbitals to form new hybrid atomic orbitals. These hybrid orbitals should be appropriate for electron pairing and forming of new bonds according to the Valence Bond Theory.
Complete answer:
We are given 5 compounds out of which three are polyatomic molecules and two are diatomic compounds. The hybridisation of polyatomic compounds can be given by the formula:
Hydridisation=2no.of valence electrons in the central atom+no.ofHydrogen Atoms+no.of Halide Atoms±Formal Charge
If the answer obtained is-
| Answer Obtained/Steric Number | Hybridisation | Geometry |
|---|---|---|
| 2 | sp | Linear |
| 3 | sp2 | Trigonal Planar |
| 4 | sp3 | Tetrahedral |
| 5 | sp3d | Trigonal Bipyramidal |
| 6 | sp3d2 | Octahedral |
Let us consider the given compounds one by one.
- CH4 : According to the formula the hybridisation can be calculated as:
Hybridisation=24+4=4
Hence the hybridisation would be sp3 with the geometry to be tetrahedral. There are zero lone pairs in the Carbon atom of the molecule hence the shape of the molecule will also be tetrahedral itself. Here the p-orbitals of C combine with four 1s orbitals of Hydrogen atoms to give four hybridised sp3 orbitals. The structure of the molecule is as follows:
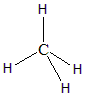
- NH3 : According to the formula the hybridisation can be calculated as:
Hybridisation=25+3=4
Hence the hybridisation would be sp3 with the geometry to be tetrahedral. There is one lone pair of Nitrogen present, occupying one side of the tetrahedron. Because of the bond pair- lone pair repulsion, the bond angle reduces and makes the shape as Pyramidal. Here the 2p orbitals of N combine with three 1s orbitals of Hydrogen making four sp3hybridised orbitals. - O2&N2 : Both these molecules are homonuclear diatomic species. Neither Oxygen nor Nitrogen has to hybridize to make bonds. The internuclear axis in these is the z-axis. For homonuclear diatomic species, the orbitals can directly combine in space. The 2px orbital can combine directly sidelong with another 2px orbital, similarly with 2py&2pz. For dioxygen, it uses its 2pz orbital to form a sigma bond, and either 2pxor 2py to form the pi bond. For dinitrogen, 2pz orbital is used for making the sigma bond, and both the 2{p_x}{\text{& }}2{p_y} to form two pi bonds; forming triple bond between two nitrogen atoms. Therefore, O2&N2 has no hybridisation.
- H2O: According to the formula the hybridisation can be calculated as:
Hybridisation=26+2=4
Hence the hybridisation would be sp3 with the geometry to be tetrahedral. There are two lone pairs of Oxygen present, occupying two sides of the tetrahedron. Because of the bond pair- lone pair repulsion, the bond angle increases and makes the shape angular. Here the 2p orbitals of O combine with two 1s orbitals of Hydrogen making three sp3 hybridised orbitals. The structure of the molecule is:
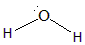
Note:
The bond order and other properties of Diatomic species can be found out with the Help of Molecular Orbital Diagrams (MODs). MODs for polyatomic species are found to be complicated to understand. If there are lone pairs present on the central atom, the Hybridisation and geometry of the molecule will remain the same, their shape will change accordingly. For example, the shape of a molecule having Tetrahedral geometry with one L.P will be Pyramidal shaped. Similarly, molecules with Trigonal Planar geometry and one L.P will have angular shape.
