Question
Question: What is the hybridization of boron in \({\left[ {{\text{B}}{{\left( {{\text{OH}}} \right)}_{\text{4}...
What is the hybridization of boron in [B(OH)4] - ?
A.sp2
B.sp3
C.sp3d2
D.None of these
Solution
Hybridization refers to the intermixing of two or more atomic orbitals which are of nearly the same energies to give rise to the formation of new orbitals called hybrid orbitals.
Hybridization takes place in the following ways: sp3 hybridization, sp2 hybridization, sp hybridization, sp3d hybridization and sp3d2 hybridization.
Boron is a second period or first row element and so it does not have vacant d orbitals to undergo sp3d2 hybridization.
Complete step by step answer:
We need to find out the hybridization of boron atom in the ion[B(OH)4] - .
When the 2s and the three 2p orbitals of boron, viz. the 2px, 2py and 2pz orbitals get intermixed, four new orbitals called thesp3 hybrid orbitals will be formed. An sp3hybridIzed orbital has s-character 25% and p-character 75%.
In sp2hybridization, the 2s orbital and two of the three 2p orbitals of boron get intermixed, while the third 2p orbital remains unchanged. A sp2hybrid orbital has 33% s-character and 66% p-character. In sp hybridization, the 2s orbital and one of the three 2p orbitals of boron get intermixed, while the remaining two 2p orbitals are left unchanged.
Now, let us study the structure of the [B(OH)4] - ion.
Boron atom has the atomic number 5 and so in the ground state has the electronic configuration 1s22s22p1 .
Boron atom in the first excited state has the electronic configuration has has the electronic configuration 1s22s12px12py1 . Thus, boron forms 4 sp3hybridized orbitals.
In the [B(OH)4] - ion, the unpaired orbitals will form 3 bonds with 3 hydroxyl groups. The vacant orbital forms a coordinate bond with the OH− group.
Thus, boron will have sp3hybridization in [B(OH)4] - and the molecule will have tetrahedral structure.
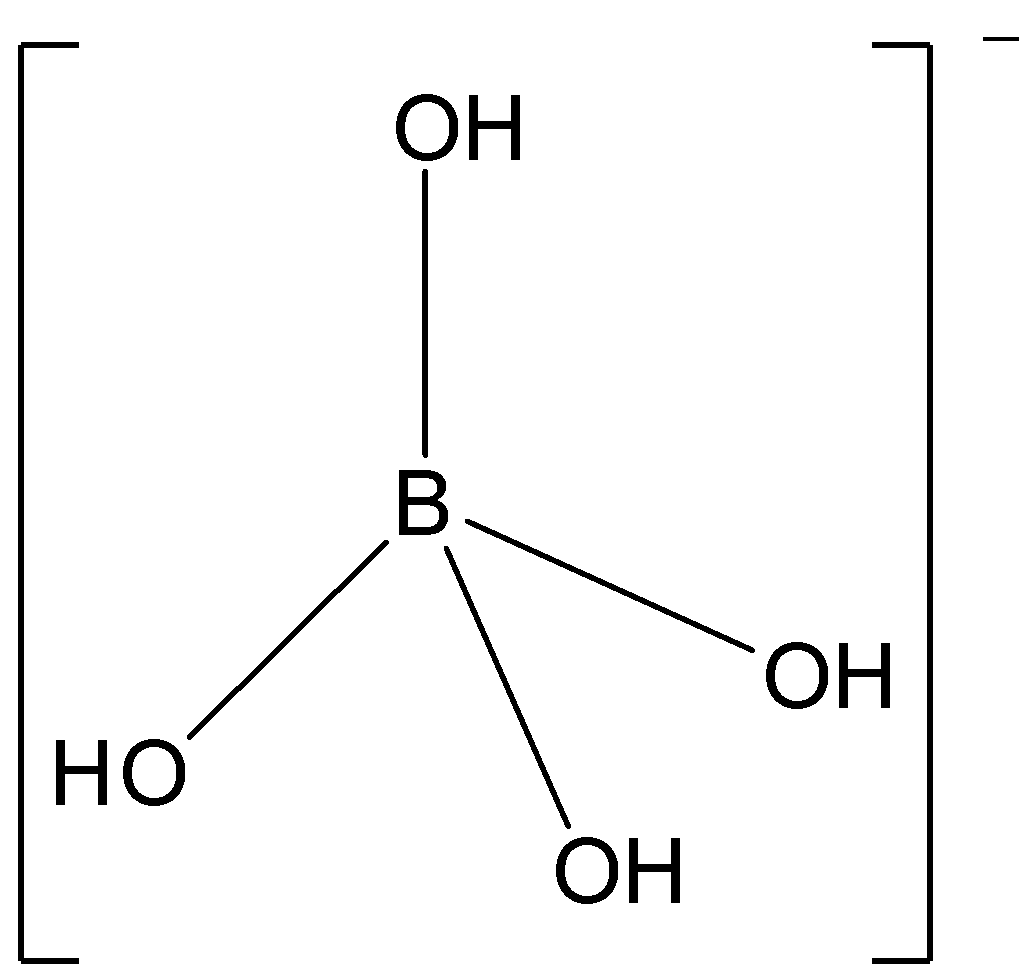
So, option B is correct.
Note:
Tetrahydroxyborate is produced when boric acid and hydroxide ions react with each other.
B(OH)3 + HO - →[B(OH)4]−
It can again capture a proton and assimilate it into the anion to form boric acid because of which it has a weak basic character.
[B(OH)4]−+H+→B(OH)3 + H2O
